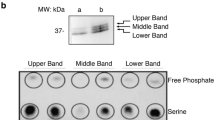
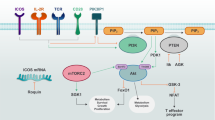
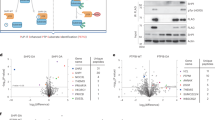
Abstract
T lymphocyte proliferation and differentiation are controlled by signaling pathways initiated by the T cell antigen receptor. Here we explore how key serine-threonine kinases and their substrates mediate T cell signaling and coordinate T cell metabolism to meet the metabolic demands of participating in an immune response.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Matthews, S.A. & Cantrell, D.A. New insights into the regulation and function of serine/threonine kinases in T lymphocytes. Immunol. Rev. 228, 241–252 (2009).
Rosse, C. et al. PKC and the control of localized signal dynamics. Nat. Rev. Mol. Cell Biol. 11, 103–112 (2010).
Weiss, A., Wiskocil, R.L. & Stobo, J.D. The role of T3 surface molecules in the activation of human T cells: a two-stimulus requirement for IL 2 production reflects events occurring at a pre-translational level. J. Immunol. 133, 123–128 (1984).
Cantrell, D.A., Davies, A.A. & Crumpton, M.J. Activators of protein kinase C down-regulate and phosphorylate the T3/T-cell antigen receptor complex of human T lymphocytes. Proc. Natl. Acad. Sci. USA 82, 8158–8162 (1985).
Monks, C.R., Kupfer, H., Tamir, I., Barlow, A. & Kupfer, A. Selective modulation of protein kinase C-θ during T-cell activation. Nature 385, 83–86 (1997).
Dustin, M.L. & Depoil, D. New insights into the T cell synapse from single molecule techniques. Nat. Rev. Immunol. 11, 672–684 (2011).
Quann, E.J., Liu, X., Altan-Bonnet, G. & Huse, M. A cascade of protein kinase C isozymes promotes cytoskeletal polarization in T cells. Nat. Immunol. 12, 647–654 (2011).
Jun, J.E., Rubio, I. & Roose, J.P. Regulation of Ras exchange factors and cellular localization of Ras activation by lipid messengers in T cells. Front. Immunol. 4, 239 (2013).
Rozengurt, E. Protein kinase D signaling: multiple biological functions in health and disease. Physiology (Bethesda) 26, 23–33 (2011).
Carrasco, S. & Merida, I. Diacylglycerol-dependent binding recruits PKCθ and RasGRP1 C1 domains to specific subcellular localizations in living T lymphocytes. Mol. Biol. Cell 15, 2932–2942 (2004).
Spitaler, M., Emslie, E., Wood, C.D. & Cantrell, D. Diacylglycerol and protein kinase D localization during T lymphocyte activation. Immunity 24, 535–546 (2006).
Huse, M. et al. Spatial and temporal dynamics of T cell receptor signaling with a photoactivatable agonist. Immunity 27, 76–88 (2007).
Quann, E.J., Merino, E., Furuta, T. & Huse, M. Localized diacylglycerol drives the polarization of the microtubule-organizing center in T cells. Nat. Immunol. 10, 627–635 (2009).
Pearce, L.R., Komander, D. & Alessi, D.R. The nuts and bolts of AGC protein kinases. Nat. Rev. Mol. Cell Biol. 11, 9–22 (2010).
Matsumoto, R. et al. Phosphorylation of CARMA1 plays a critical role in T cell receptor-mediated NF-κB activation. Immunity 23, 575–585 (2005).
Dustin, M.L. PKC-θ: hitting the bull's eye. Nat. Immunol. 12, 1031–1032 (2011).
Liu, X., Kapoor, T.M., Chen, J.K. & Huse, M. Diacylglycerol promotes centrosome polarization in T cells via reciprocal localization of dynein and myosin II. Proc. Natl. Acad. Sci. USA 110, 11976–11981 (2013).This article, together with refs. 7 and 13, provides evidence that DAG gradients generated at the immunological synapse promote the recruitment of different PKC isozymes and the microtubule-organizing center and links these phenomena, identifying the myosin regulatory light chain as the PKC substrate relevant in the regulation of the localization of the nonmuscle myosin motor complex.
Ma, J.S.Y., Haydar, T.F. & Radoja, S. Protein kinase C-δ localizes to secretory lysosomes in CD8+ CTL and directly mediates TCR signals leading to granule exocytosis-mediated cytotoxicity. J. Immunol. 181, 4716–4722 (2008).
Letschka, T. et al. PKC-θ selectively controls the adhesion-stimulating molecule Rap1. Blood 112, 4617–4627 (2008).
Fagerholm, S., Morrice, N., Gahmberg, C.G. & Cohen, P. Phosphorylation of the cytoplasmic domain of the integrin CD18 chain by protein kinase C isoforms in leukocytes. J. Biol. Chem. 277, 1728–1738 (2002).
Matthews, S.A., Rozengurt, E. & Cantrell, D. Protein kinase D. A selective target for antigen receptors and a downstream target for protein kinase C in lymphocytes. J. Exp. Med. 191, 2075–2082 (2000).
Waldron, R.T. et al. Activation loop Ser744 and Ser748 in protein kinase D are transphosphorylated in vivo. J. Biol. Chem. 276, 32606–32615 (2001).
Rey, O., Reeve, J.R., Zhukova, E., Sinnett-Smith, J. & Rozengurt, E. G protein–coupled receptor-mediated phosphorylation of the activation loop of protein kinase D: dependence on plasma membrane translocation and protein kinase Cɛ. J. Biol. Chem. 279, 34361–34372 (2004).
Matthews, S.A. et al. Unique functions for protein kinase D1 and protein kinase D2 in mammalian cells. Biochem. J. 432, 153–163 (2010).
Navarro, M.N. et al. Protein kinase D2 has a restricted but critical role in T-cell antigen receptor signalling in mature T-cells. Biochem. J. 442, 649–659 (2012).
Fischer, A.M., Katayama, C.D., Pagès, G., Pouysségur, J. & Hedrick, S.M. The role of erk1 and erk2 in multiple stages of T cell development. Immunity 23, 431–443 (2005).
D'Souza, W.N., Chang, C.-F., Fischer, A.M., Li, M. & Hedrick, S.M. The Erk2 MAPK regulates CD8 T cell proliferation and survival. J. Immunol. 181, 7617–7629 (2008).
Costello, P.S., Nicolas, R.H., Watanabe, Y., Rosewell, I. & Treisman, R. Ternary complex factor SAP-1 is required for Erk-mediated thymocyte positive selection. Nat. Immunol. 5, 289–298 (2004).
Wagner, E.F. & Eferl, R. Fos/AP-1 proteins in bone and the immune system. Immunol. Rev. 208, 126–140 (2005).
Owens, D.M. & Keyse, S.M. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene 26, 3203–3213 (2007).
Lovrić, J., Dammeier, S., Kieser, A., Mischak, H. & Kolch, W. Activated raf induces the hyperphosphorylation of stathmin and the reorganization of the microtubule network. J. Biol. Chem. 273, 22848–22855 (1998).
Lin, J.-X., Spolski, R. & Leonard, W.J. Critical role for Rsk2 in T-lymphocyte activation. Blood 111, 525–533 (2008).
Romeo, Y., Zhang, X. & Roux, P.P. Regulation and function of the RSK family of protein kinases. Biochem. J. 441, 553–569 (2012).
Fukunaga, R. & Hunter, T. MNK1, a new MAP kinase-activated protein kinase, isolated by a novel expression screening method for identifying protein kinase substrates. EMBO J. 16, 1921–1933 (1997).
Waskiewicz, A.J., Flynn, A., Proud, C.G. & Cooper, J.A. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J. 16, 1909–1920 (1997).
Ueda, T. et al. Combined deficiency for MAP kinase-interacting kinase 1 and 2 (Mnk1 and Mnk2) delays tumor development. Proc. Natl. Acad. Sci. USA 107, 13984–13990 (2010).
Okkenhaug, K., Bilancio, A., Emery, J.L. & Vanhaesebroeck, B. Phosphoinositide 3-kinase in T cell activation and survival. Biochem. Soc. Trans. 32, 332–335 (2004).
Costello, P.S., Gallagher, M. & Cantrell, D.A. Sustained and dynamic inositol lipid metabolism inside and outside the immunological synapse. Nat. Immunol. 3, 1082–1089 (2002).
Harriague, J. & Bismuth, G. Imaging antigen-induced PI3K activation in T cells. Nat. Immunol. 3, 1090–1096 (2002).
Huppa, J.B., Gleimer, M., Sumen, C. & Davis, M.M. Continuous T cell receptor signaling required for synapse maintenance and full effector potential. Nat. Immunol. 4, 749–755 (2003).
Garçon, F. et al. CD28 provides T-cell costimulation and enhances PI3K activity at the immune synapse independently of its capacity to interact with the p85/p110 heterodimer. Blood 111, 1464–1471 (2008).
Calleja, V. et al. Intramolecular and intermolecular interactions of protein kinase B define its activation in vivo. PLoS Biol. 5, e95 (2007).
Bayascas, J.R. et al. Mutation of the PDK1 PH domain inhibits protein kinase B/Akt, leading to small size and insulin resistance. Mol. Cell. Biol. 28, 3258–3272 (2008).This article describes the generation of knock-in mice expressing a PDK1 mutant incapable of binding PtdIns(3,4,5)P 3 , and demonstrates that PDK1 binding to PtdIns(3,4,5)P 3 is required for efficient Akt activation, but some Akt activation is detected in absence of PDK1-PtdIns(3,4,5)P 3 binding.
Najafov, A., Shpiro, N. & Alessi, D.R. Akt is efficiently activated by PIF-pocket- and PtdIns(3,4,5)P3-dependent mechanisms leading to resistance to PDK1 inhibitors. Biochem. J. 448, 285–295 (2012).Together with ref. 42, this article provides evidence of an alternative mechanism for Akt activation by PDK1 that is independent of PDK1-PtdIns(3,4,5)P 3 binding. This work shows how mTORC2-mediated phosphorylation of Ser473 in Akt recruits PDK1 via a substrate-docking motif termed the PIF-pocket, allowing phosphorylation of Akt Thr308 by PDK1 and efficient Akt activation.
Sengupta, S., Peterson, T.R. & Sabatini, D.M. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol. Cell 40, 310–322 (2010).
García-Martínez, J.M. & Alessi, D.R. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1). Biochem. J. 416, 375–385 (2008).
Heikamp, E.B. et al. The AGC kinase SGK1 regulates TH1 and TH2 differentiation downstream of the mTORC2 complex. Nat. Immunol. 15, 457–464 (2014).
Wu, C. et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 496, 513–517 (2013).
Kleinewietfeld, M. et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 496, 518–522 (2013).
Delgoffe, G.M. et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat. Immunol. 12, 295–303 (2011).This article provides key evidence about the specific role of mTORC1 and mTORC2 complexes in T cell function. In Rheb-deficient mice with disabled mTORC1 function, T H 1 and T H 17 responses are impaired both in vitro and in vivo ; in RICTOR-deficient mice in which mTORC2 function is ablated, T cells fail to generate T H 2 responses.
Waugh, C., Sinclair, L., Finlay, D., Bayascas, J.R. & Cantrell, D. Phosphoinositide (3,4,5)-triphosphate binding to phosphoinositide-dependent kinase 1 regulates a protein kinase B/Akt signaling threshold that dictates T-cell migration, not proliferation. Mol. Cell. Biol. 29, 5952–5962 (2009).
Sarbassov, D.D., Guertin, D.A., Ali, S.M. & Sabatini, D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307, 1098–1101 (2005).
Barata, J.T. et al. Activation of PI3K is indispensable for interleukin 7–mediated viability, proliferation, glucose use, and growth of T cell acute lymphoblastic leukemia cells. J. Exp. Med. 200, 659–669 (2004).
Cornish, G.H., Sinclair, L.V. & Cantrell, D.A. Differential regulation of T-cell growth by IL-2 and IL-15. Blood 108, 600–608 (2006).
Brunn, G.J. et al. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J. 15, 5256–5267 (1996).
Jacobs, M.D. et al. Pim-1 ligand-bound structures reveal the mechanism of serine/threonine kinase inhibition by LY294002. J. Biol. Chem. 280, 13728–13734 (2005).
Bain, J. et al. The selectivity of protein kinase inhibitors: a further update. Biochem. J. 408, 297–315 (2007).
Macintyre, A.N. et al. Protein kinase B controls transcriptional programs that direct cytotoxic T cell fate but is dispensable for T cell metabolism. Immunity 34, 224–236 (2011).This work provides key evidence that Akt does not mediate T cell metabolic responses. Using mice deficient in PI3K and PDK1 function and Akt-specific pharmacologic inhibitors, the authors show that Akt catalytic activity controls expression of key effector molecules but is not required for glucose uptake or IL-2–mediated proliferation.
Eijkelenboom, A. & Burgering, B.M.T. FOXOs: signalling integrators for homeostasis maintenance. Nat. Rev. Mol. Cell Biol. 14, 83–97 (2013).
Fabre, S. et al. FOXO1 regulates L-Selectin and a network of human T cell homing molecules downstream of phosphatidylinositol 3-kinase. J. Immunol. 181, 2980–2989 (2008).
Kerdiles, Y.M. et al. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat. Immunol. 10, 176–184 (2009).
Ouyang, W. et al. Novel Foxo1-dependent transcriptional programs control T(reg) cell function. Nature 491, 554–559 (2012).
Ouyang, W., Beckett, O., Flavell, R.A. & Li, M.O. An essential role of the Forkhead-box transcription factor Foxo1 in control of T cell homeostasis and tolerance. Immunity 30, 358–371 (2009).
Carlson, C.M. et al. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature 442, 299–302 (2006).
Finlay, D.K. et al. Phosphoinositide-dependent kinase 1 controls migration and malignant transformation but not cell growth and proliferation in PTEN-null lymphocytes. J. Exp. Med. 206, 2441–2454 (2009).
Preston, G.C., Feijoo-Carnero, C., Schurch, N., Cowling, V.H. & Cantrell, D.A. The impact of KLF2 modulation on the transcriptional program and function of CD8 T cells. PLoS ONE 8, e77537 (2013).
Takada, K. et al. Kruppel-like factor 2 is required for trafficking but not quiescence in postactivated T cells. J. Immunol. 186, 775–783 (2011).
Cyster, J.G. & Schwab, S.R. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu. Rev. Immunol. 30, 69–94 (2012).
Sinclair, L.V. et al. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat. Immunol. 9, 513–521 (2008).
Hand, T.W. et al. Differential effects of STAT5 and PI3K/AKT signaling on effector and memory CD8 T-cell survival. Proc. Natl. Acad. Sci. USA 107, 16601–16606 (2010).
Wang, R. & Green, D.R. Metabolic checkpoints in activated T cells. Nat. Immunol. 13, 907–915 (2012).
Marko, A.J., Miller, R.A., Kelman, A. & Frauwirth, K.A. Induction of glucose metabolism in stimulated T lymphocytes is regulated by mitogen-activated protein kinase signaling. PLoS ONE 5, e15425 (2010).
Carr, E.L. et al. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J. Immunol. 185, 1037–1044 (2010).
Wang, R. et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 35, 871–882 (2011).This article shows how TCR signaling induces metabolic reprogramming in naive T cells. In this work, the authors show that acute deletion of the transcription factor c-Myc inhibits TCR-mediated glycolysis and glutaminolysis, impairing T cell proliferation. Together with refs. 72 and 73 , this study shows that activation of the Erk1 and Erk2 kinases regulates the expression of c-Myc.
Sinclair, L.V. et al. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat. Immunol. 10.1038/ni.2556 (2013).
Bar-Peled, L. & Sabatini, D.M. Regulation of mTORC1 by amino acids. Trends Cell Biol. (2014).
Dibble, C.C. & Manning, B.D. Signal integration by mTORC1 coordinates nutrient input with biosynthetic output. Nat. Cell Biol. 15, 555–564 (2013).
Powell, J.D. & Delgoffe, G.M. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity 33, 301–311 (2010).
Ricoult, S.J.H. & Manning, B.D. The multifaceted role of mTORC1 in the control of lipid metabolism. EMBO Rep. 14, 242–251 (2013).
Kidani, Y. et al. Sterol regulatory element-binding proteins are essential for the metabolic programming of effector T cells and adaptive immunity. Nat. Immunol. 14, 489–499 (2013).
Finlay, D.K. et al. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J. Exp. Med. 209, 2441–2453 (2012).
Shi, L.Z. et al. HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med. 208, 1367–1376 (2011).This work shows that T H 17 differentiation requires the upregulation of glycolytic activity and that the mTORC1-regulated expression of the transcription factor HIF1α is a crucial mediator of glycolytic pathways. Together with ref. 81 this article defines key role for mTORC1-HIF1α axis in the maintenance of glucose metabolism and glycolysis in effector T cells.
Wang, H. et al. Negative regulation of Hif1a expression and TH17 differentiation by the hypoxia-regulated microRNA miR-210. Nat. Immunol. 15, 393–401 (2014).
Shimobayashi, M. & Hall, M.N. Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat. Rev. Mol. Cell Biol. 15, 155–162 (2014).
Cham, C.M. & Gajewski, T.F. Glucose availability regulates IFN-γ production and p70S6 kinase activation in CD8+ effector T cells. J. Immunol. 174, 4670–4677 (2005).This article shows how glucose availability controls key effector functions, such as IFN-γ production, in CD8 T cells. This work also shows that glucose deprivation decreases the phosphorylation of the mTORC1 substrate p70S6 kinase.
Nakaya, M. et al. Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity (2014).
Tamás, P. et al. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J. Exp. Med. 203, 1665–1670 (2006).
Hardie, D.G., Ross, F.A. & Hawley, S.A. AMP-activated protein kinase: a target for drugs both ancient and modern. Chem. Biol. 19, 1222–1236 (2012).
Tamás, P. et al. LKB1 is essential for the proliferation of T-cell progenitors and mature peripheral T cells. Eur. J. Immunol. 40, 242–253 (2010).
Rolf, J. et al. AMPKα1: A glucose sensor that controls CD8 T-cell memory. Eur. J. Immunol. 43, 889–896 (2013).
Cai, S.-L. et al. Activity of TSC2 is inhibited by AKT-mediated phosphorylation and membrane partitioning. J. Cell Biol. 173, 279–289 (2006).
Menon, S. et al. Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. Cell 156, 771–785 (2014).
Yang, K., Neale, G., Green, D.R., He, W. & Chi, H. The tumor suppressor Tsc1 enforces quiescence of naive T cells to promote immune homeostasis and function. Nat. Immunol. 12, 888–897 (2011).This article describes the role of TSC1-dependent control of mTOR using Tsc1-deficient mice. Tsc1-deficient T cells had more mTORC1 activity, causing the disruption of immune homeostasis.
Efeyan, A. & Sabatini, D.M. Nutrients and growth factors in mTORC1 activation. Biochem. Soc. Trans. 41, 902–905 (2013).
Gwinn, D.M. et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 30, 214–226 (2008).
Araki, K. et al. mTOR regulates memory CD8 T-cell differentiation. Nature 460, 108–112 (2009).This work reports how, unexpectedly, the mTORC1 pharmacological inhibitor rapamycin enhances the generation of CD8 memory T cells. The regulation of mTORC1 activity appears to be a control switch for effector-to-memory transition in CD8 T cells.
Navarro, M.N., Goebel, J., Feijoo-Carnero, C., Morrice, N. & Cantrell, D.A. Phosphoproteomic analysis reveals an intrinsic pathway for the regulation of histone deacetylase 7 that controls the function of cytotoxic T lymphocytes. Nat. Immunol. 12, 352–361 (2011).This article describes the application of large-scale quantitative phosphoproteomic approaches to analyze the TCR-regulated phosphoproteome of primary cytotoxic T cells. This work reports the existence of a basal web protein phosphorylation and also affords new insights about the scope of TCR-regulated phosphorylations.
Ruperez, P., Gago-Martinez, A., Burlingame, A.L. & Oses-Prieto, J.A. Quantitative phosphoproteomic analysis reveals a role for serine and threonine kinases in the cytoskeletal reorganization in early T cell receptor activation in human primary T cells. Mol. Cell. Proteomics 11, 171–186 (2012).
Mayya, V. et al. Quantitative phosphoproteomic analysis of T cell receptor signaling reveals system-wide modulation of protein-protein interactions. Sci. Signal. 2, ra46 (2009).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Navarro, M., Cantrell, D. Serine-threonine kinases in TCR signaling. Nat Immunol 15, 808–814 (2014). https://doi.org/10.1038/ni.2941
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2941
This article is cited by
-
Fatty acid metabolism of immune cells: a new target of tumour immunotherapy
Cell Death Discovery (2024)
-
Characterization of the effect of histone deacetylation inhibitors on CD8+ T cells in the context of aging
Journal of Translational Medicine (2022)
-
Fatty Acid Metabolism and Cancer Immunotherapy
Current Oncology Reports (2022)
-
N-myristoyltransferase deficiency impairs activation of kinase AMPK and promotes synovial tissue inflammation
Nature Immunology (2019)
-
The dark side of PD-1 receptor inhibition
Nature (2017)