Abstract
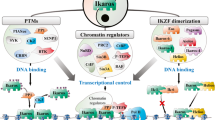
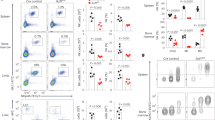
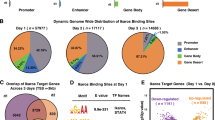
C2H2 zinc fingers are found in several key transcriptional regulators in the immune system. However, these proteins usually contain more fingers than are needed for sequence-specific DNA binding, which suggests that different fingers regulate different genes and functions. Here we found that mice lacking finger 1 or finger 4 of Ikaros exhibited distinct subsets of the hematological defects of Ikaros-null mice. Most notably, the two fingers controlled different stages of lymphopoiesis, and finger 4 was selectively required for tumor suppression. The distinct defects support the hypothesis that only a small number of genes that are targets of Ikaros are critical for each of its biological functions. The subcategorization of functions and target genes by mutagenesis of individual zinc fingers will facilitate efforts to understand how zinc-finger transcription factors regulate development, immunity and disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Wang, J.H. et al. Selective defects in the development of the fetal and adult lymphoid system in mice with an Ikaros null mutation. Immunity 5, 537–549 (1996).
Georgopoulos, K. Haematopoietic cell-fate decisions, chromatin regulation and ikaros. Nat. Rev. Immunol. 2, 162–174 (2002).
Yoshida, T., Ng, S.Y. & Georgopoulos, K. Awakening lineage potential by Ikaros-mediated transcriptional priming. Curr. Opin. Immunol. 22, 154–160 (2010).
Yoshida, T., Ng, S.Y., Zuniga-Pflucker, J.C. & Georgopoulos, K. Early hematopoietic lineage restrictions directed by Ikaros. Nat. Immunol. 7, 382–391 (2006).
Kirstetter, P., Thomas, M., Dierich, A., Kastner, P. & Chan, S. Ikaros is critical for B cell differentiation and function. Eur. J. Immunol. 32, 720–730 (2002).
Thompson, E.C. et al. Ikaros DNA-binding proteins as integral components of B cell developmental-stage-specific regulatory circuits. Immunity 26, 335–344 (2007).
Reynaud, D. et al. Regulation of B cell fate commitment and immunoglobulin heavy-chain gene rearrangements by Ikaros. Nat. Immunol. 9, 927–936 (2008).
Trageser, D. et al. Pre-B cell receptor-mediated cell cycle arrest in Philadelphia chromosome-positive acute lymphoblastic leukemia requires IKAROS function. J. Exp. Med. 206, 1739–1753 (2009).
Ma, S. et al. Ikaros and Aiolos inhibit pre-B-cell proliferation by directly suppressing c-Myc expression. Mol. Cell Biol. 30, 4149–4158 (2010).
Winandy, S., Wu, P. & Georgopoulos, K. A dominant mutation in the Ikaros gene leads to rapid development of leukemia and lymphoma. Cell 83, 289–299 (1995).
Dumortier, A. et al. Notch activation is an early and critical event during T-cell leukemogenesis in Ikaros-deficient mice. Mol. Cell Biol. 26, 209–220 (2006).
Mullighan, C.G. et al. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature 453, 110–114 (2008).
Mullighan, C.G. et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N. Engl. J. Med. 360, 470–480 (2009).
Kim, J. et al. Ikaros DNA-binding proteins direct formation of chromatin remodeling complexes in lymphocytes. Immunity 10, 345–355 (1999).
Sridharan, R. & Smale, S.T. Predominant interaction of both Ikaros and Helios with the NuRD complex in immature thymocytes. J. Biol. Chem. 282, 30227–30238 (2007).
Zhang, J. et al. Harnessing of the nucleosome-remodeling-deacetylase complex controls lymphocyte development and prevents leukemogenesis. Nat. Immunol. 13, 86–94 (2011).
Harker, N. et al. The CD8α gene locus is regulated by the Ikaros family of proteins. Mol. Cell 10, 1403–1415 (2002).
Naito, T., Gomez-Del Arco, P., Williams, C.J. & Georgopoulos, K. Antagonistic interactions between Ikaros and the chromatin remodeler Mi-2β determine silencer activity and Cd4 gene expression. Immunity 27, 723–734 (2007).
Gomez-del Arco, P. et al. Alternative promoter usage at the Notch1 locus supports ligand-independent signaling in T cell development and leukemogenesis. Immunity 33, 685–698 (2010).
Ng, S.Y., Yoshida, T., Zhang, J. & Georgopoulos, K. Genome-wide lineage-specific transcriptional networks underscore Ikaros-dependent lymphoid priming in hematopoietic stem cells. Immunity 30, 493–507 (2009).
John, L.B. & Ward, A.C. The Ikaros gene family: transcriptional regulators of hematopoiesis and immunity. Mol. Immunol. 48, 1272–1278 (2011).
Sun, L., Liu, A. & Georgopoulos, K. Zinc finger-mediated protein interactions modulate Ikaros activity, a molecular control of lymphocyte development. EMBO J. 15, 5358–5369 (1996).
Trinh, L.A. et al. Down-regulation of TDT transcription in CD4+CD8+ thymocytes by Ikaros proteins in direct competition with an Ets activator. Genes Dev. 15, 1817–1832 (2001).
McCarty, A.S., Kleiger, G., Eisenberg, D. & Smale, S.T. Selective dimerization of a C2H2 zinc finger subfamily. Mol. Cell 11, 459–470 (2003).
Tupler, R., Perini, G. & Green, M.R. Expressing the human genome. Nature 409, 832–833 (2001).
Ravasi, T. et al. Systematic characterization of the zinc-finger-containing proteins in the mouse transcriptome. Genome Res. 13, 1430–1442 (2003).
Klug, A. The discovery of zinc fingers and their applications in gene regulation and genome manipulation. Annu. Rev. Biochem. 79, 213–231 (2010).
Wolfe, S.A., Nekludova, L. & Pabo, C.O. DNA recognition by Cys2His2 zinc finger proteins. Annu. Rev. Biophys. Biomol. Struct. 29, 183–212 (2000).
Molnar, A. & Georgopoulos, K. The Ikaros gene encodes a family of functionally diverse zinc finger DNA-binding proteins. Mol. Cell Biol. 14, 8292–8303 (1994).
Cobb, B.S. et al. Targeting of Ikaros to pericentromeric heterochromatin by direct DNA binding. Genes Dev. 14, 2146–2160 (2000).
Koipally, J., Heller, E.J., Seavitt, J.R. & Georgopoulos, K. Unconventional potentiation of gene expression by Ikaros. J. Biol. Chem. 277, 13007–13015 (2002).
Hahm, K. et al. Helios, a T cell-restricted Ikaros family member that quantitatively associates with Ikaros at centromeric heterochromatin. Genes Dev. 12, 782–796 (1998).
Payne, K.J. et al. Ikaros isoform x is selectively expressed in myeloid differentiation. J. Immunol. 170, 3091–3098 (2003).
Miller, J., McLachlan, A.D. & Klug, A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 4, 1609–1614 (1985).
Shastry, B.S. Transcription factor IIIA (TFIIIA) in the second decade. J. Cell Sci. 109, 535–539 (1996).
Filippova, G.N. et al. An exceptionally conserved transcriptional repressor, CTCF, employs different combinations of zinc fingers to bind diverged promoter sequences of avian and mammalian c-myc oncogenes. Mol. Cell Biol. 16, 2802–2813 (1996).
Ohlsson, R., Renkawitz, R. & Lobanenkov, V. CTCF is a uniquely versatile transcription regulator linked to epigenetics and disease. Trends Genet. 17, 520–527 (2001).
Renda, M. et al. Critical DNA binding interactions of the insulator protein CTCF: a small number of zinc fingers mediate strong binding, and a single finger-DNA interaction controls binding at imprinted loci. J. Biol. Chem. 282, 33336–33345 (2007).
Nurmemmedov, E., Yengo, R.K., Uysal, H., Karlsson, R. & Thunnissen, M.M. New insights into DNA-binding behavior of Wilms tumor protein (WT1)–a dual study. Biophys. Chem. 145, 116–125 (2009).
Nakahashi, H. et al. A genome-wide map of CTCF multivalency redefines the CTCF code. Cell Rep. 3, 1678–1689 (2013).
Hardy, R.R., Carmack, C.E., Shinton, S.A., Kemp, J.D. & Hayakawa, K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J. Exp. Med. 173, 1213–1225 (1991).
Rolink, A., Grawunder, U., Winkler, T.H., Karasuyama, H. & Melchers, F. IL-2 receptor α chain (CD25, TAC) expression defines a crucial stage in pre-B cell development. Int. Immunol. 6, 1257–1264 (1994).
Rothenberg, E.V., Moore, J.E. & Yui, M.A. Launching the T-cell-lineage developmental programme. Nat. Rev. Immunol. 8, 9–21 (2008).
Randall, T.D., Carragher, D.M. & Rangel-Moreno, J. Development of secondary lymphoid organs. Annu. Rev. Immunol. 26, 627–650 (2008).
Juric, D. et al. Differential gene expression patterns and interaction networks in BCR-ABL-positive and -negative adult acute lymphoblastic leukemias. J. Clin. Oncol. 25, 1341–1349 (2007).
Lu, Q. delta-Catenin dysregulation in cancer: interactions with E-cadherin and beyond. J. Pathol. 222, 119–123 (2010).
Wang, H. et al. The role of Crk/Dock180/Rac1 pathway in the malignant behavior of human ovarian cancer cell SKOV3. Tumour Biol. 31, 59–67 (2010).
Perentes, J.Y. et al. Cancer cell-associated MT1-MMP promotes blood vessel invasion and distant metastasis in triple-negative mammary tumors. Cancer Res. 71, 4527–4538 (2011).
Marcais, A. et al. Genetic inactivation of Ikaros is a rare event in human T-ALL. Leuk. Res. 34, 426–429 (2010).
Wong, S. et al. Sole BCR-ABL inhibition is insufficient to eliminate all myeloproliferative disorder cell populations. Proc. Natl. Acad. Sci. USA 101, 17456–17461 (2004).
Papathanasiou, P. et al. Widespread failure of hematolymphoid differentiation caused by a recessive niche-filling allele of the Ikaros transcription factor. Immunity 19, 131–144 (2003).
Lickwar, C.R., Mueller, F., Hanlon, S.W., McNally, J.G. & Lieb, J.D. Genome-wide protein-DNA binding dynamics suggest a molecular clutch for transcription factor function. Nature 484, 251–255 (2012).
Virely, C. et al. Haploinsufficiency of the IKZF1 (IKAROS) tumor suppressor gene cooperates with BCR-ABL in a transgenic model of acute lymphoblastic leukemia. Leukemia 24, 1200–1204 (2010).
Sun, Z. et al. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science 288, 2369–2373 (2000).
Montecino-Rodriguez, E., Leathers, H. & Dorshkind, K. Identification of a B-1 B cell-specified progenitor. Nat. Immunol. 7, 293–301 (2006).
Aliahmad, P., de la Torre, B. & Kaye, J. Shared dependence on the DNA-binding factor TOX for the development of lymphoid tissue-inducer cell and NK cell lineages. Nat. Immunol. 11, 945–952 (2010).
Pandya-Jones, A. & Black, D.L. Co-transcriptional splicing of constitutive and alternative exons. RNA 15, 1896–1908 (2009).
Nagalakshmi, U., Waern, K. & Snyder, M. RNA-Seq: a method for comprehensive transcriptome analysis. Curr. Protoc. Mol. Biol. 89, 4.11.1–4.11.13 (2010).
Giardine, B. et al. Galaxy: a platform for interactive large-scale genome analysis. Genome Res. 15, 1451–1455 (2005).
Trapnell, C., Pachter, L. & Salzberg, S.L. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25, 1105–1111 (2009).
Blankenberg, D. et al. Galaxy: a web-based genome analysis tool for experimentalists. Curr. Protoc. Mol. Biol. 89, 19.10.1–19–10–21 (2010).
Fujita, P.A. et al. The UCSC Genome Browser database: update 2011. Nucleic Acids Res. 39, D876–D882 (2010).
Goecks, J., Nekrutenko, A. & Taylor, J. Galaxy: a comprehensive approach for supporting accessible, reproducible, and transparent computational research in the life sciences. Genome Biol. 11, R86 (2010).
Kent, W.J. et al. The human genome browser at UCSC. Genome Res. 12, 996–1006 (2002).
Mortazavi, A., Williams, B.A., McCue, K., Schaeffer, L. & Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 5, 621–628 (2008).
Anders, S. & Huber, W. Differential expression analysis for sequence count data. Genome Biol. 11, R106 (2010).
Eisen, M.B., Spellman, P.T., Brown, P.O. & Botstein, D. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 95, 14863–14868 (1998).
de Hoon, M.J., Imoto, S., Nolan, J. & Miyano, S. Open source clustering software. Bioinformatics 20, 1453–1454 (2004).
Saldana, A.J. Java Treeview - extensible visualization of microarray data. Bioinformatics 20, 3246–3248 (2004).
Thomas, P.D. et al. Applications for protein sequence-function evolution data: mRNA/protein expression analysis and coding SNP scoring tools. Nucleic Acids Res. 34, W645–W650 (2006).
O'Geen, H., Frietze, S. & Farnham, P.J. Using ChIP-seq technology to identify targets of zinc finger transcription factors. Methods Mol. Biol. 649, 437–455 (2010).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009).
Blahnik, K.R. et al. Sole-Search: an integrated analysis program for peak detection and functional annotation using ChIP-seq data. Nucleic Acids Res. 38, e13 (2010).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Bailey, T.L. et al. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 37, W202–W208 (2009).
Acknowledgements
We thank P. Aliahmad, D. Bhatt, K. Dorshkind, C. Li and E. Montecino-Rodriguez for advice and/or critical reading of the manuscript; the Division of Laboratory Animal Medicine of the University of California, Los Angeles, for ongoing care of mice; and H. Mak, T. Jacob, C. Garcia, J. Flores and J. Lorenzano for assistance with the mouse colony. ChIP-seq and RNA-Seq libraries were sequenced at the Epigenome Data Production Facility of the University of Southern California, and the Broad Stem Cell Research Center High Throughput Sequencing Core of the University of California, Los Angeles. Supported by the US National Institutes of Health (RO1DK043726 to S.T.S. and U54HG004558 to P.J.F.). O.N.W. is an Investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
H.S., J.M., T.L.A., S.F., D.C., S.E.W. and G.W.L. designed and did experiments and analyzed data; S.J.B. provided intellectual input and experimental advice; P.J.F., O.N.W. and S.T.S. supervised research and analyzed data; and H.S. and S.T.S. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Tables 1–2 (PDF 6921 kb)
Rights and permissions
About this article
Cite this article
Schjerven, H., McLaughlin, J., Arenzana, T. et al. Selective regulation of lymphopoiesis and leukemogenesis by individual zinc fingers of Ikaros. Nat Immunol 14, 1073–1083 (2013). https://doi.org/10.1038/ni.2707
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2707
This article is cited by
-
The transcription factor Aiolos restrains the activation of intestinal intraepithelial lymphocytes
Nature Immunology (2024)
-
IKAROS and AIOLOS directly regulate AP-1 transcriptional complexes and are essential for NK cell development
Nature Immunology (2024)
-
IKAROS: from chromatin organization to transcriptional elongation control
Cell Death & Differentiation (2023)
-
T-ALL can evolve to oncogene independence
Leukemia (2021)
-
Ikaros family zinc-finger 1 mutation is an independent factor for the poor prognosis of adult B-cell acute lymphoblastic leukemia, and allogeneic hematopoietic stem cell transplantation can improve clinical outcomes
Bone Marrow Transplantation (2019)