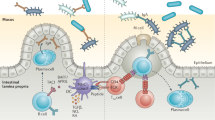
Abstract
The mammalian gastrointestinal tract, the site of digestion and nutrient absorption, harbors trillions of beneficial commensal microbes from all three domains of life. Commensal bacteria, in particular, are key participants in the digestion of food, and are responsible for the extraction and synthesis of nutrients and other metabolites that are essential for the maintenance of mammalian health. Many of these nutrients and metabolites derived from commensal bacteria have been implicated in the development, homeostasis and function of the immune system, suggesting that commensal bacteria may influence host immunity via nutrient- and metabolite-dependent mechanisms. Here we review the current knowledge of how commensal bacteria regulate the production and bioavailability of immunomodulatory, diet-dependent nutrients and metabolites and discuss how these commensal bacteria–derived products may regulate the development and function of the mammalian immune system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214 (2012).
Qin, J. et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65 (2010).
Semova, I. et al. Microbiota regulate intestinal absorption and metabolism of fatty acids in the zebrafish. Cell Host Microbe 12, 277–288 (2012).
Shin, S.C. et al. Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science 334, 670–674 (2011).
Bäckhed, F. et al. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 101, 15718–15723 (2004).
Ley, R.E., Turnbaugh, P.J., Klein, S. & Gordon, J.I. Microbial ecology: human gut microbes linked to obesity. Nature 444, 1022–1023 (2006).
Bäckhed, F., Ley, R.E., Sonnenburg, J.L., Peterson, D.A. & Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 307, 1915–1920 (2005).
Hooper, L.V., Midtvedt, T. & Gordon, J.I. Host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu. Rev. Nutr. 22, 283–307 (2002).
Flint, H.J., Scott, K.P., Louis, P. & Duncan, S.H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 9, 577–589 (2012).
Kau, A.L., Ahern, P.P., Griffin, N.W., Goodman, A.L. & Gordon, J.I. Human nutrition, the gut microbiome and immune system. Nature 474, 327–336 (2011).
Musso, G., Gambino, R. & Cassader, M. Interactions between gut microbiota and host metabolism predisposing to obesity. Annu. Rev. Med. 62, 361–380 (2011).
Nicholson, J.K. et al. Host-gut microbiota metabolic interactions. Science 336, 1262–1267 (2012).
Holmes, E., Li, J.V., Athanasiou, T., Ashrafian, H. & Nicholson, J.K. Understanding the role of gut microbiome-host metabolic signal disruption in health and disease. Trends Microbiol. 19, 349–359 (2011).
Tremaroli, V. & Bächked, F. Functional interactions between the gut microbiota and host metabolism. Nature 489, 242–249 (2012).
Hill, D.A. & Artis, D. Intestinal bacteria and the regulation of immune cell homeostasis. Annu. Rev. Immunol. 28, 623–667 (2010).
Round, J.L. & Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 9, 313–323 (2009).
Littman, D.R. & Pamer, E.G. Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microbe 10, 311–323 (2011).
Chinen, T. & Rudensky, A.Y. The effects of commensal microbiota on immune cell subsets and inflammatory responses. Immunol. Rev. 245, 45–55 (2012).
Honda, K. & Littman, D.R. The microbiome in infectious disease and inflammation. Annu. Rev. Immunol. 30, 759–795 (2012).
Hooper, L.V., Littman, D.R. & Macpherson, A.J. Interactions between the microbiota and the immune system. Science 336, 1268–1273 (2012).
Molloy, M.J., Bouladoux, N. & Belkaid, Y. Intestinal microbiota: shaping local and systemic immune responses. Semin. Immunol. 24, 58–66 (2012).
Abt, M.C. & Artis, D. The dynamic influence of commensal bacteria on the immune response to pathogens. Curr. Opin. Microbiol. 16, 4–9 (2013).
Kamada, N., Seo, S., Chen, G.Y. & Núñez, G. Role of gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 13, 321–335 (2013).
Abraham, C. & Medzhitov, R. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenerology 140, 1729–1737 (2011).
Wang, R. & Green, D.R. Metabolic checkpoints in activated T cells. Nat. Immunol. 13, 907–915 (2012).
Pearce, E.L. & Pearce, E.J. Metabolic pathways in immune cell activation and quiescence. Immunity 38, 633–643 (2013).
Michalek, R.D. et al. Cutting Edge: Distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 186, 3299–3303 (2011).
Shi, L.Z. et al. HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of Th17 and Treg cells. J. Exp. Med. 208, 1367–1376 (2011). References 27 and 28 demonstrate that distinct metabolic programs critically regulate differentiation of T cell subsets.
Haschemi, A. et al. The sedoheptulose kinase CARKL directs macrophage polarization through control of glucose metabolism. Cell Metab. 15, 813–826 (2012).
Donohoe, D.R., Wali, A., Brylawski, B.P. & Bultman, S.J. Microbial regulation of glucose metabolism and cell-cycle progression in mammalian coloncytes. PLoS ONE 7, e46589 (2012).
Odegaard, J.I. & Chawla, A. The immune system as a sensor of the metabolic state. Immunity 38, 644–654 (2013).
Thomas, C., Pellicciari, R., Pruzanski, M., Auwerx, J. & Schoonjans, K. Targeting bile-acid signaling for metabolic diseases. Nat. Rev. Drug Discov. 7, 678–693 (2008).
Fiorucci, S., Mencarelli, A., Palladino, G. & Cipriani, S. Bile-acid-activated receptors: targeting TGR5 and farnesoid-X-receptor in lipid and glucose disorders. Trends Pharmacol. Sci. 30, 570–580 (2009).
Ridlon, J.M., Kang, D.L. & Hylemon, P.B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 47, 241–259 (2006).
Trauner, M. & Boyer, J.L. Bile salt transporters: molecular characterization, function, and regulation. Physiol. Rev. 83, 633–671 (2003).
Tanaka, H., Hashiba, H., Kok, J. & Mierau, I. Bile salt hydrolase of Bifidobacterium longum–biochemical and genetic characterization. Appl. Environ. Microbiol. 66, 2502–2512 (2000).
Jones, B.V., Begley, M., Hill, C., Gahan, C.G. & Marchesi, J.R. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc. Natl. Acad. Sci. USA 105, 13580–13585 (2008).
Sayin, S.I. et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 17, 225–235 (2013). This article comprehensively characterizes bile acid metabolism in multiple mouse tissues and provides insight into how beneficial commensal bacteria in the intestine regulate metabolism of bile acids.
Martin, F.P. et al. A top-down systems biology view of microbiome-mammalian metabolic interactions in a mouse model. Mol. Syst. Biol. 3, 112 (2007).
Claus, S.P. et al. Systemic multicompartmental effects of the gut microbiome on mouse metabolic phenotypes. Mol. Syst. Biol. 4, 219 (2008).
Martin, F.P. et al. Panorganismal gut microbiome-host metabolic crosstalk. J. Proteome Res. 8, 2090–2105 (2009).
Martin, F.P. et al. Dietary modulation of gut functional ecology studied by fecal metabonomics. J. Proteome Res. 9, 5284–5295 (2010).
Swann, J.R. et al. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc. Natl. Acad. Sci. USA 108, 4523–4530 (2011). This article comprehensively characterizes amounts of bile acid metabolites in multiple tissues of germ-free mice versus conventionally reared mice.
Claus, S.P. et al. Colonization-induced host-gut microbial metabolic interaction. MBio 2, e00271–10 (2011). References 39–42 and 44 compare metabolite levels in multiple compartments of conventionally reared mice versus germ-free mice using metabolomic approaches.
Duboc, H. et al. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut 62, 531–539 (2013).
Vavassori, P., Mencarelli, A., Renga, B., Distrutti, E. & Fiorucci, S. The bile acid receptor FXR is a modulator of intestinal innate immunity. J. Immunol. 183, 6251–6261 (2009).This article demonstrates that FXR regulates intestinal inflammation in a model of IBD and provides mechanistic insight into how bile acid–FXR signaling inhibits activity of NF-κB.
Wang, Y.D., Chen, W.D., Yu, D., Forman, B.M. & Huang, W. The G-protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulated hepatic inflammatory response through antagonizing nuclear factor κ light-chain enhancer of activated B cells (NF-κB) in mice. Hepatology 54, 1421–1432 (2011).
Pols, T.W. et al. TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab. 14, 747–757 (2011). This article demonstrates that the bile acid receptor TGR5 attenuates atherosclerosis by decreasing macrophage-associated inflammation.
Maruyama, T. et al. Identification of membrane-type receptor for bile acids (M-BAR). Biochem. Biophys. Res. Commun. 298, 714–719 (2002).
Pellicciari, R. et al. Discovery of 6alpha-ethyl-23(S)-methylcholic acid (S-EMCA, INT-777) as a potent and selective agonist for the TGR5 receptor, a novel target for diabesity. J. Med. Chem. 52, 7958–7961 (2009).
David, M., Petricoin, E. III & Larner, A.C. Activation of protein kinase A inhibits interferon induction of the Jak/Stat pathway in U266 cells. J. Biol. Chem. 271, 4585–4588 (1996).
Lee, E.H. & Rikihisa, Y. Protein kinase A-mediated inhibition of gamma interferon-induced tyrosine phosphorylation of Janus kinases and latent cytoplasmic transcription factors in human monocytes by Ehrlichia chaffeensis. Infect. Immun. 66, 2514–2520 (1998).
Wen, A.Y., Sakamoto, K.M. & Miller, L.S. The role of the transcription factor CREB in immune function. J. Immunol. 185, 6413–6419 (2010).
Cipriani, S. et al. The bile acid receptor GPBAR-1 (TGR5) modulates integrity of intestinal barrier and immune response to experimental colitis. PLoS ONE 6, e25637 (2011).
Gadaleta, R.M. et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut 60, 463–472 (2011).
Mencarelli, A. et al. The bile acid sensor farnesoid X receptor is a modulator of liver immunity in a rodent model of acute hepatitis. J. Immunol. 183, 6657–6666 (2009).
Diao, H. et al. Osteopontin as a mediator of NKT cell function in T cell-mediated liver diseases. Immunity 21, 539–550 (2004).
Lenz, K. Bile acid metabolism and vitamin B12 absorption in ulcerative colitis. Scand. J. Gastroenterol. 11, 769–775 (1976).
Rutgeerts, P., Ghoos, Y. & Vantrappen, G. Bile acid studies in patients with Crohn's colitis. Gut 20, 1072–1077 (1979).
Turnbaugh, P.J. et al. A core gut microbiome in obese and lean twins. Nature 457, 480–484 (2009).
Turnbaugh, P.J. et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006).
Cani, P.D. et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57, 1470–1481 (2008).
Kobayashi, M. et al. Prevention and treatment of obesity, insulin resistance, and diabetes by bile acid-binding resin. Diabetes 56, 239–247 (2007).
Henao-Mejia, J. et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 482, 179–185 (2012).
Qin, J. et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490, 55–60 (2012).
Karlsson, F.H. et al. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat. Commun. 3, 1245 (2012).
Koren, O. et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc. Natl. Acad. Sci. USA 108, 4592–4598 (2011).
Sobhani, I. et al. Microbial dysbiosis in colorectal cancer (CRC) patients. PLoS ONE 6, e16393 (2011).
Abt, M.C. et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity 37, 158–170 (2012).
Ganal, S.C. et al. Priming of natural killer cells by nonmucosal mononuclear phagocytes requires instructive signals from commensal microbiota. Immunity 37, 171–186 (2012).References 69 and 70 demonstrate that commensal bacteria–derived signals regulate antiviral immunity.
Renga, B. et al. The acid sensor FXR is required for immune-regulatory activities of TLR-9 in intestinal inflammation. PLoS ONE 8, e54472 (2013).
Nijmeijer, R.M. et al. Farnesoid X receptor (FXR) activation and FXR genetic variation in inflammatory bowel disease. PLoS ONE 6, e23745 (2011).
Devkota, S. et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature 487, 104–108 (2012).This reference demonstrates that at least some bile acids promote outgrowth of a pathogenic bacterial species in IL-10–deficient mice.
Chang, K.O. et al. Bile acids are essential for porcine enteric calicivirus replication in association with down-regulation of signal transducer and activator of transcription 1. Proc. Natl. Acad. Sci. USA 101, 8733–8738 (2004).
Chang, K.O. & George, D.W. Bile acids promote the expression of hepatitis C virus in replicon-harboring cells. J. Virol. 81, 9633–9640 (2007). References 74 and 75 demonstrate that bile acids regulate viral replication.
Miller, T.L. & Wolin, M.J. Fermentations by saccharolytic intestinal bacteria. Am. J. Clin. Nutr. 32, 164–172 (1979).
Cummings, J.H. Fermentation in the human large intestine: evidence and implications for health. Lancet 1, 1206–1209 (1983).
Cummings, J.H. & Macfarlane, G.T. The control and consequences of fermentation in the human colon. J. Appl. Bacteriol. 70, 443–459 (1991).
Wong, J.M.W., de Souza, R., Kendall, C.W.C., Emam, A. & Jenkins, D.J.A. Colonic health: fermentation and short chain fatty acids. J. Clin. Gastroenterol. 40, 235–243 (2006).
Cummings, J.H., Pomare, E.W., Branch, W.J., Naylor, C.P. & Macfarlane, G.T. Short chain fatty acids in human large intestine, portal, hepatic, and venous blood. Gut 28, 1221–1227 (1987).
Macfarlane, S. & Macfarlane, G.T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 62, 67–72 (2003).
Smiricky-Tjardes, M.R. et al. In vitro fermentation characteristics of selected oligosaccharides by swine fecal microflora. J. Anim. Sci. 81, 2505–2514 (2003).
Høverstad, T. & Midtvedt, T. Short-chain fatty acids in germfree mice and rats. J. Nutr. 116, 1772–1776 (1986).
Donohoe, D.R. et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 13, 517–526 (2011). This article demonstrates that commensal bacteria–derived butyrate, an SCFA, is critical for maintaining metabolic homeostasis and regulating autophagy in colonocytes.
Maslowski, K.M. et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461, 1282–1286 (2009). This article demonstrates that commensal bacteria–derived SCFAs have an anti-inflammatory role in a model of IBD.
Bjursell, M. et al. Improved glucose control and reduced body fat mass in free fatty acid receptor 2-deficient mice fed a high-fat diet. Am. J. Physiol. Endocrinol. Metab. 300, E211–E220 (2011).
Bellahcene, M. et al. Male mice that lack the G-protein-coupled receptor GPR41 have low energy expenditure and increased body fat content. Br. J. Nutr. 109, 1755–1764 (2012).
Kimura, I. et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc. Natl. Acad. Sci. USA 108, 8030–8035 (2011).
Sina, C., Jiang, H.-P., Li, J, Schreiber, S. & Rosenstiel, P. G protein-coupled receptor 43 is essential for neutrophil recruitment during intestinal inflammation. J. Immunol. 183, 7514–7522 (2009).
Vinolo, M.A. et al. SCFAs induce mouse neutrophil chemotaxis through the GPR43 receptor. PLoS ONE 6, e21205 (2011).
Brown, A.J. et al. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain fatty acids. J. Biol. Chem. 278, 11312–11319 (2003).
Le Poul, E. et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 278, 25481–25489 (2003).
Nilsson, N.E., Kotarsky, K., Owman, C. & Olde, B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem. Biophys. Res. Commun. 303, 1047–1052 (2003). References 91–93 provide comprehensive pharmacologic characterizations of SCFA-GPR41 and SCFA-GPR43 interactions and demonstrate that SCFAs regulate immune cells.
Cousens, L.S., Gallwitz, D. & Alberts, B.M. Different accessibilities in chromatin to histone acetylase. J. Biol. Chem. 254, 1716–1723 (1979).
Donohoe, D.R. et al. The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol. Cell 48, 612–626 (2012).
Hinnebusch, B.F., Meng, S., Wu, J.T., Archer, S.Y. & Hodin, R.A. The effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation. J. Nutr. 132, 1012–1017 (2002).
Waldecker, M., Kautenburger, T., Daumann, H., Busch, C. & Schrenk, D. Inhibition of histone-deacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon. J. Nutr. Biochem. 19, 587–593 (2008).
Virgin, H.W. & Levine, B. Autophagy genes in immunity. Nat. Immunol. 10, 461–470 (2009).
Hudson, B.D., Tikhonova, I.G., Pandey, S.K., Ulven, T. & Milligan, G. Extracellular ionic locks determine variation in constitutive activity and ligand potency between species orthologs of the free fatty acid receptors FFA2 and FFA3. J. Biol. Chem. 287, 41195–41209 (2012).
Cox, M.A. et al. Short-chain fatty acids act as anti-inflammatory mediators by regulating prostaglandin E(2) and cytokines. World J. Gastroenterol. 15, 5549–5557 (2009).
Venkatraman, A. et al. Amelioration of dextran sulfate colitis by butyrate: role of heat shock protein 70 and NF-κB. Am. J. Physiol. Gastroenterol. Liver Physiol. 285, G177–G184 (2003).
Berndt, B.E. et al. Butyrate increases IL-23 production by stimulated dendritic cells. Am. J. Physiol. Gastrointest. Liver Physiol. 303, G1384–G1392 (2012).
Liu, L. et al. Butyrate interferes with the differentiation and function of human monocyte-derived dendritic cells. Cell Immunol. 277, 66–73 (2012).
Eftimiadi, C. et al. Divergent effect of the anaerobic bacteria by-product butyric acid on the immune response: suppression of T-lymphocyte proliferation and stimulation of interleuking-1 beta production. Oral Microbiol. Immunol. 6, 17–23 (1991).
Gilbert, K.M., DeLoose, A., Valentine, J.L. & Fifer, E.K. Structure-activity relationship between carboxylic acids and T cell cycle blockade. Life Sci. 78, 2159–2165 (2006).
Bailón, E. et al. Butyrate in vitro immune-modulatory effects might be mediated through a proliferation-related induction of apoptosis. Immunobiology 215, 863–873 (2010).
Zimmerman, M.A. et al. Butyrate suppresses colonic inflammation through HDAC1-dependent Fas upregulation and Fas-mediated apoptosis of T cells. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G1405–G1415 (2012).
Huang, N., Katz, J.P., Martin, D.R. & Wu, G.D. Inhibition of IL-8 gene expression in Caco-2 cells by compounds which induce histone hyperacetylation. Cytokine 9, 27–36 (1997).
Patel, K.K. & Stappenbeck, T.S. Autophagy and intestinal homeostasis. Annu. Rev. Physiol. 75, 241–262 (2012).
Shakespear, M.R., Halili, M.A., Irvine, K.M., Fairlie, D.P. & Sweet, M.J. Histone deacetylases as regulators of inflammation and immunity. Trends Immunol. 32, 335–343 (2011).
Scheppach, W. et al. Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterology 103, 51–56 (1992).
Segain, J.P. et al. Buytrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn's disease. Gut 47, 397–403 (2000).
Resta, S.C. Effects of probiotics and commensals on intestinal epithelial physiology: implications for nutrient handling. J. Physiol. (Lond.) 587, 4169–4174 (2009).
Bhaskaram, P. Micronutrient malnutrition, infection, and immunity: an overview. Nutr. Rev. 60, S40–S45 (2002).
Cheng, C.H., Chang, S.J., Lee, B.J., Lin, K.L. & Huang, Y.C. Vitamin B6 supplementation increases immune responses in critically ill patients. Eur. J. Clin. Nutr. 60, 1207–1213 (2006).
Meydani, S.N. et al. Vitamin E supplementation and in vivo immune response in healthy elderly subjects: a randomized controlled trial. J. Am. Med. Assoc. 277, 1380–1386 (1997).
Tamura, J. et al. Immunomodulation by vitamin B12: augmentation of CD8+ T lymphocytes and natural killer (NK) cell activity in vitamin B12-deficient patients by methyl-B12 treatment. Clin. Exp. Immunol. 116, 28–32 (1999).
Cantorna, M.T., Zhu, Y., Froicu, M. & Wittke, A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am. J. Clin. Nutr. 80, 1717S–1720S (2004).
Hashimoto, T. et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 487, 477–481 (2012). This article suggests that commensal bacteria may regulate intestinal inflammation by influencing absorption of amino acids.
Kunisawa, J., Hashimoto, E., Ishikawa, I. & Kiyono, H. A pivotal role of vitamin B9 in the maintenance of regulatory T cells in vitro and in vivo. PLoS ONE 7, e32094 (2012).
Spencer, S.P. & Belkaid, Y. Dietary and commensal derived nutrients: shaping mucosal and systemic immunity. Curr. Opin. Immunol. 24, 379–384 (2012).
Kjer-Nielsen, L. et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 491, 717–723 (2012)This article demonstrates that B-vitamin metabolites bind MR1 and promote mucosa-associated invariant T cell activation.
Dusseaux, M. et al. Human MAIT cells are xenobiotic resistant, tissue-targeted, CD161hi IL-17 secreting T cells. Blood 117, 1250–1259 (2011).
Walker, L.J. et al. Human MAIT cells and CD8alphaalpha cells develop from a pool of type-17 precommitted CD8+ T cells. Blood 119, 422–433 (2012).
Le Bourhis, L. et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat. Immunol. 11, 701–708 (2010).
Le Bourhis, L., Mburu, Y.K. & Lantz, O. MAIT cells, surveyors of a new class of antigen: development and functions. Curr. Opin. Immunol. 25, 174–180 (2013).
Smith, M.I., et al. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science 339, 548–554 (2013).
Trehan, I. et al. Antibiotics as part of the management of severe acute malnutrition. N. Engl. J. Med. 368, 425–435 (2013).
Mestdagh, R. et al. Gut microbiota modulate the metabolism of brown adipose tissue in mice. J. Proteome Res. 11, 620–630 (2012).
Tannahill, G.M. et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496, 238–242 (2013). This article demonstrates that glucose oxidation and amounts of the citric acid cycle intermediate succinate regulate production of IL-1β.
Matsumoto, M. et al. Impact of intestinal microbiota on intestinal luminal metabolome. Scientific Reports 2, 233 (2012).
Whitt, D.D. & Demoss, R.D. Effect of microflora on the free amino acid distribution in various regions of the mouse gastrointestinal tract. Appl. Microbiol. 30, 609–615 (1975).
McGaha, T.L. et al. Amino acid catabolism: a pivotal regulator of innate and adaptive immunity. Immunol. Rev. 249, 135–157 (2012).
Morris, S.M. Jr. Arginases and arginine deficiency syndromes. Curr. Opin. Clin. Nutr. Metab. Care 15, 64–70 (2012).
Puccetti, P. & Grohmann, U. IDO and regulatory T cells: a role for reverse signaling and non-canonical NF-κB activation. Nat. Rev. Immunol. 7, 817–823 (2007).
Das, P., Lahiri, A., Lahiri, A. & Chakravortty, D. Modulation of the arginase pathway in the context of microbial pathogenesis: a metabolic enzyme moonlighting as an immune modulator. PLoS Pathog. 6, e1000899 (2010).
Munn, D.H. et al. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J. Exp. Med. 189, 1363–1372 (1999).
Nowak, E.C. et al. Tryptophan hydroxylase-1 regulates immune tolerance and inflammation. J. Exp. Med. 209, 2127–2135 (2012).
Rodriguez, P.C. et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 64, 5839–5849 (2004).
Cobbold, S.P. et al. Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc. Natl. Acad. Sci. USA 106, 12055–12060 (2009).
Scrimshaw, N.S., Wilson, D. & Bressani, R. Infection and kwaszhiorkor. J. Trop. Pediatr. 6, 37–43 (1960).
Müller, O. & Krawinkel, M. Malnutrition and health in developing countries. CMAJ 173, 279–286 (2005).
Black, R.E. et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 371, 243–260 (2008).
Rice, A.L., Sacco, L., Hyder, A. & Black, R.E. Malnutrition as an underlying cause of childhood deaths associated with infectious diseases in developing countries. Bull. World Health Organ. 78, 1207–1221 (2000).
Pretorius, P.J. & De Villers, L.S. Antibody response in children with protein malnutrition. Am. J. Clin. Nutr. 10, 379–383 (1962).
Savy, M. et al. Landscape analysis of interactions between nutrition and vaccine responses in children. J. Nutr. 139, 2154S–2218S (2009).
Dumas, M.E. et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc. Natl. Acad. Sci. USA 103, 12511–12516 (2006).
Rossjohn, J., Pellicci, D.G., Patel, O., Gapin, L. & Godfrey, D.I. Recognition of CD1d-restricted antigens by natural killer T cells. Nat. Rev. Immunol. 12, 845–857 (2012).
Wei, B. et al. Commensal microbiota and CD8+ T cells shape the formation of invariant NKT cells. J. Immunol. 184, 1218–1226 (2010).
Olszak, T. et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science 336, 489–493 (2012).
Kidani, Y. & Bensinger, S.J. Liver X receptor and peroxisome proliferator-activated receptor as integrators of lipid homeostasis and immunity. Immunol. Rev. 249, 72–83 (2012).
Hong, C. et al. Coordinate regulation of neutrophil homeostasis by liver X receptors in mice. J. Clin. Invest. 122, 337–347 (2012).
Odegaard, J.I. et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 447, 1116–1120 (2007).
Odegaard, J.I. et al. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 7, 496–507 (2008).
Mukundan, L. et al. PPAR-δ senses and orchestrates clearance of apoptotic cells to promote tolerance. Nat. Med. 15, 1266–1272 (2009).
Kelly, D. et al. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-γ and RelA. Nat. Immunol. 5, 104–112 (2004).
Are, A. et al. Enterococcus faecalis from newborn babies regulate endogenous PPARgamma activity and IL-10 levels in colonic epithelial cells. Proc. Natl. Acad. Sci. USA 105, 1943–1948 (2008).
Gerriets, V.A. & Rathmell, J.C. Metabolic pathways in T cell fate and function. Trends Immunol. 33, 168–173 (2012).
Pearce, E.L. et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460, 103–107 (2009). This article suggests that metabolism of fatty acids is critical for formation of CD8+ memory T cells.
Ito, K. et al. PML-PPAR-δ pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nat. Med. 18, 1350–1358 (2012).
Acknowledgements
We thank all members of the Artis laboratory for discussions and critical reading of the manuscript. Supported by US National Institutes of Health grants (AI061570, AI074878, AI083480, AI087990, AI095466, AI095608, AI097333 and AI102942 to D.A.), the Burroughs Wellcome Fund Investigator in Pathogenesis of Infectious Disease Award (D.A.) and the Crohn's and Colitis Foundation of America (D.A.). J.R.B. is supported by National Institutes of Health grant T32-AI060516.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Brestoff, J., Artis, D. Commensal bacteria at the interface of host metabolism and the immune system. Nat Immunol 14, 676–684 (2013). https://doi.org/10.1038/ni.2640
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2640
This article is cited by
-
Survival, immune response, and gut microbiota in Litopenaeus vannamei fed with synbiotics and postbiotics and challenged with Vibrio parahaemolyticus
Aquaculture International (2024)
-
The interactions between the host immunity and intestinal microorganisms in fish
Applied Microbiology and Biotechnology (2024)
-
Intratumoural microbiota: from theory to clinical application
Cell Communication and Signaling (2023)
-
Short-term feeding of defatted bovine colostrum mitigates inflammation in the gut via changes in metabolites and microbiota in a chicken animal model
Animal Microbiome (2023)
-
Genes mcr improve the intestinal fitness of pathogenic E. coli and balance their lifestyle to commensalism
Microbiome (2023)