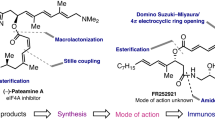
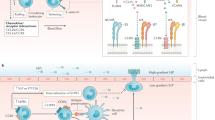
Abstract
Gastrointestinal diseases can result from the inadequate or excessive response of the immune system to self or innocuous antigens. Moreover, the physiologic activation of the immune system against non-self antigens is a major clinical problem in liver organ transplantation. At present, many drugs are available that suppress the activation of the immune system, although most of the currently used immunosuppressive drugs lack specificity in terms of their molecular targets and, therefore, have the potential to generate numerous side effects. The advances that have been made in understanding the molecular events that underlie the activation of the immune system have led to the development of a new generation of 'small molecules' that are endowed with immunosuppressive properties and can serve as immunomodulatory agents. Among these new small molecules, inhibitors of Janus kinase 3, p21-Rac1 and p38 mitogen-activated protein kinase represent the most innovative approach to immunosuppression, and could be a promising alternative to current immunosuppressive therapies. Here, we report on the progress that has been made in the development of small molecules in the field of gastroenterology.
Key Points
-
Current immunosuppressive drugs have a wide range of effects, most of them undesirable
-
The discovery of intracellular pathways involved in immune system activation (e.g. JAK–STAT and p38–NFκB pathways) has provided potential new targets for immunosuppression
-
The ability to produce numerous, new, small molecules offers the possibility to obtain new specific inhibitors of key molecules involved in immune system activation
-
The discovery of new compounds that specifically target key molecules involved in immune system activation will provide new classes of immunosuppressive agents endowed with low toxicity and reduced side effects
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Neurath MF et al. (2002) The role of TH1/TH2 polarization in mucosal immunity. Nat Med 8: 567–573
Hanauer SB and Present DH (2003) The state of the art in the management of inflammatory bowel disease. Rev Gastroenterol Disord 3: 81–92
Hong JC and Kahan BD (2000) Immunosuppressive agents in organ transplantation: past, present, and future. Semin Nephrol 20: 108–125
Kino T et al. (1987) FK-506, a novel immunosuppressant isolated from a Streptomyces. II. Immunosuppressive effect of FK-506 in vitro. J Antibiot (Tokyo) 40: 1256–1265
Tanaka H et al. (1987) Physicochemical properties of FK-506, a novel immunosuppressant isolated from Streptomyces tsukubaensis. Transplant Proc 19: 11–16
Harding MW et al. (1989) A receptor for the immunosuppressant FK506 is a cis-trans peptidyl-prolyl isomerase. Nature 341: 758–760
Shaw JP et al. (1988) Identification of a putative regulator of early T cell activation genes. Science 241: 202–205
Crabtree GR (1989) Contingent genetic regulatory events in T lymphocyte activation. Science 243: 355–361
Lichtiger S et al. (1994) Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med 330: 1841–1845
Svanoni F et al. (1998) Effectiveness of cyclosporine A (CsA) in the treatment of active refractory ulcerative colitis (UC) [abstract]. Gastroenterology 114: A1096
Van Assche G et al. (2003) Randomized, double-blind comparison of 4 mg/kg versus 2 mg/g intravenous cyclosporine in severe ulcerative colitis. Gastroenterology 125: 1025–1031
McDonald JW et al. (2005) Cyclosporine for induction of remission in Crohn's disease. The Cochrane Database of Systematic Reviews, Art. No CD000297
Hogenauer C et al. (2003) Effect of oral tacrolimus (FK 506) on steroid-refractory moderate/severe ulcerative colitis. Aliment Pharmacol Ther 18: 415–423
Baumgart DC, et al. (2003) Rescue therapy with tacrolimus is effective in patients with severe and refractory inflammatory bowel disease. Aliment Pharmacol Ther 17: 1273–1281
Lucey MR et al. (2005) A comparison of tacrolimus and cyclosporine in liver transplantation: effects on renal function and cardiovascular risk status. Am J Transplant 5: 1111–1119
Dumont FJ (2004) ISAtx-247 (Isotechnika/Roche). Curr Opin Investig Drugs 5: 542–550
Corral LG and Kaplan G (1999) Immunomodulation by thalidomide and thalidomide analogs. Ann Rheum Dis 58 (Suppl 1): I107–I113
Corral LG et al. (1996) Selection of novel analogs of thalidomide with enhanced tumor necrosis factor alpha inhibitory activity. Mol Med 2: 506–515
Corral LG et al. (1999) Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-α. J Immunol 163: 380–386
Geitz H et al. (1996) Thalidomide selectively modulates the density of cell surface molecules involved in the adhesion cascade. Immunopharmacology 31: 213–221
Bauditz J et al. (2002) Thalidomide reduces tumour necrosis factor alpha and interleukin 12 production in patients with chronic active Crohn's disease. Gut 50: 196–200
Abraham RT (1998) Mammalian target of rapamycin: immunosuppressive drugs uncover a novel pathway of cytokine receptor signaling. Curr Opin Immunol 10: 330–336
Calne RY et al. (1989) Rapamycin for immunosuppression in organ allografting. Lancet 2: 227
Kahan BD (1992) Cyclosporin A, FK506, rapamycin: the use of a quantitative analytic tool to discriminate immunosuppressive drug interactions. J Am Soc Nephrol 2 (Suppl): S222–S227
Sanchez EQ et al. (2005) Sirolimus conversion after liver transplantation: improvement in measured glomerular filtration rate after 2 years. Transplant Proc 37: 4416–4423
Farkas S et al. (2006) Rapamycin decreases leukocyte migration in vivo and effectively reduces experimentally induced chronic colitis. Int J Colorectal Dis [doi:10.1007/s00384-005-0793-7]
Lee SK and Surh CD (2005) Role of interleukin-7 in bone and T-cell homeostasis. Immunol Rev 208: 169–180
Sandrini S (2005) Use of IL-2 receptor antagonists to reduce delayed graft function following renal transplantation: a review. Clin Transplant 19: 705–710
Becker C et al. (2005) Stepwise regulation of TH1 responses in autoimmunity: IL-12-related cytokines and their receptors. Inflamm Bowel Dis 11: 755–764
Leonard WJ et al. (1994) Sharing of a common gamma chain, gamma c, by the IL-2, IL-4, and IL-7 receptors: implications for X-linked severe combined immunodeficiency (XSCID). Adv Exp Med Biol 365: 225–232
Asao H et al. (2001) Cutting edge: the common gamma-chain is an indispensable subunit of the IL-21 receptor complex. J Immunol 167: 1–5
Leonard WJ and O'Shea JJ (1998) JAKs and STATs: biological implications. Annu Rev Immunol 16: 293–322
Cetkovic-Cvrlje M et al. (2001) Targeting Janus kinase 3 to attenuate the severity of acute graft-versus-host disease across the major histocompatibility barrier in mice. Blood 98: 1607–1613
Sudbeck EA et al. (1999) Structure-based design of specific inhibitors of Janus kinase 3 as apoptosis-inducing antileukemic agents. Clin Cancer Res 5: 1569–1582
Uckun FM et al. (2002) Janus kinase 3 inhibitor WHI-P131/JANEX-1 prevents graft-versus-host disease but spares the graft-versus-leukemia function of the bone marrow allografts in a murine bone marrow transplantation model. Blood 99: 4192–4199
Cetkovic-Cvrlje M et al. (2003) Targeting JAK3 with JANEX-1 for prevention of autoimmune type 1 diabetes in NOD mice. Clin Immunol 106: 213–225
Linwong W et al. (2005) Inhibition of the antigen-induced activation of rodent mast cells by putative Janus kinase 3 inhibitors WHI-P131 and WHI-P154 in a Janus kinase 3-independent manner. Br J Pharmacol 145: 818–828
Malavija R et al. (2001) In vivo pharmacokinetics and anti-anaphylactic activity of the novel mast cell inhibitor 4-(4'-hydroxylphenyl)-amino-6,7-dimethoxyquinazoline (WHI-P131). Am J Ther 8: 35–39
Meydan N et al. (1996) Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature 379: 645–648
Kirken RA et al. (1999) Tyrphostin AG-490 inhibits cytokine-mediated JAK3/STAT5a/b signal transduction and cellular proliferation of antigen-activated human T cells. J Leukoc Biol 65: 891–899
Wang LH et al. (1999) JAK3, STAT, and MAPK signaling pathways as novel molecular targets for the tyrphostin AG-490 regulation of IL-2-mediated T cell response. J Immunol 162: 3897–3904
Behbod F et al. (2001) Concomitant inhibition of Janus kinase 3 and calcineurin-dependent signaling pathways synergistically prolongs the survival of rat heart allografts. J Immunol 166: 3724–3732
Stepkowski SM et al. (2005) The Mannich base NC1153 promotes long-term allograft survival and spares the recipient from multiple toxicities. J Immunol 175: 4236–4246
Changelian PS et al. (2003) Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science 302: 875–878
Borie DC et al. (2005) Immunosuppression by the JAK3 inhibitor CP-690,550 delays rejection and significantly prolongs kidney allograft survival in nonhuman primates. Transplantation 79: 791–801
Paniagua R et al. (2005) Effects of JAK3 inhibition with CP-690,550 on immune cell populations and their functions in nonhuman primate recipients of kidney allografts. Transplantation 80: 1283–1292
Kudlacz E et al. (2004) The novel JAK-3 inhibitor CP-690550 is a potent immunosuppressive agent in various murine models. Am J Transplant 4: 51–57
Mortellaro A et al. (1999) New immunosuppressive drug PNU156804 blocks IL-2-dependent proliferation and NF-κB and AP-1 activation. J Immunol 162: 7102–7109
Stepkowski SM et al. (2001) PNU156804 inhibits Jak3 tyrosine kinase and rat heart allograft rejection. Transplant Proc 33: 3272–3273
Stepkowski SM et al. (2002) Selective inhibitor of Janus tyrosine kinase 3, PNU156804, prolongs allograft survival and acts synergistically with cyclosporine but additively with rapamycin. Blood 99: 680–689
Prajapati DN et al. (2003) Leflunomide treatment of Crohn's disease patients intolerant to standard immunomodulator therapy. J Clin Gastroenterol 37: 125–128
Siemasko K et al. (1998) Inhibition of JAK3 and STAT6 tyrosine phosphorylation by the immunosuppressive drug leflunomide leads to a block in IgG1 production. J Immunol 160: 1581–1588
Brown GR et al. (2000) Naphthyl ketones: a new class of Janus kinase 3 inhibitors. Bioorg Med Chem Lett 10: 575–579
Tak PP and Firestein GS (2001) NF-κB: a key role in inflammatory diseases. J Clin Invest 107: 7–11
Lee JC et al. (1994) A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372: 739–746
Kyriakis JM and Avruch J (2001) Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev 81: 807–869
Pearson G et al. (2001) Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22: 153–183
Raingeaud J et al. (1996) MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol 16: 1247–1255
Lee JC et al. (1999) p38 mitogen-activated protein kinase inhibitors—mechanisms and therapeutic potentials. Pharmacol Ther 82: 389–397
Kaminska B (2005) MAPK signaling pathways as molecular targets for anti-inflammatory therapy—from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta 1754: 253–262
Waetzig GH et al. (2002) p38 mitogen-activated protein kinase is activated and linked to TNF-α signaling in inflammatory bowel disease. J Immunol 168: 5342–5351
Hollenbach E et al. (2004) Inhibition of p38 MAP kinase- and RICK/NF-κB-signaling suppresses inflammatory bowel disease. FASEB J 18: 1550–1552
Badger AM et al. (1996) Pharmacological profile of SB 203580, a selective inhibitor of cytokine suppressive binding protein/p38 kinase, in animal models of arthritis, bone resorption, endotoxin shock and immune function. J Pharmacol Exp Ther 279: 1453–1461
Bianchi M et al. (1995) An inhibitor of macrophage arginine transport and nitric oxide production (CNI-1493) prevents acute inflammation and endotoxin lethality. Mol Med 1: 254–266
Bianchi M et al. (1996) Suppression of proinflammatory cytokines in monocytes by a tetravalent guanylhydrazone. J Exp Med 183: 927–936
Hommes D et al. (2002) Inhibition of stress-activated MAP kinases induces clinical improvement in moderate to severe Crohn's disease. Gastroenterology 122: 7–14
Regan J et al. (2003) Structure-activity relationships of the p38α MAP kinase inhibitor 1-(5-tert-butyl-2-p-tolyl-2H-pyrazol-3-yl)-3-[4-(2-morpholin-4-yl-ethoxy)naphthalen-1-yl]urea (BIRB 796). J Med Chem 46: 4676–4686
Branger J et al. (2002) Anti-inflammatory effects of a p38 mitogen-activated protein kinase inhibitor during human endotoxemia. J Immunol 168: 4070–4047
Brahn E et al. (2002) Inhibition of collagen-induced arthritis with an inhibitor of p38 MAP kinase [abstract]. Arthritis Rheum 46 (Suppl): S566
Haddad JJ (2001) VX-745. Vertex Pharmaceuticals. Curr Opin Investig Drugs 2: 1070–1076
Anselmo DM et al. (2002) FTY720 pretreatment reduces warm hepatic ischemia reperfusion injury through inhibition of T-lymphocyte infiltration. Am J Transplant 2: 843–849
Poppe D et al. (2006) Azathioprine suppresses ezrin-radixin-moesin-dependent T cell-APC conjugation through inhibition of Vav guanosine exchange activity on Rac proteins. J Immunol 176: 640–651
Tiede I et al. (2003) CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest 111: 1133–1145
Gorelik L et al. (2000) Cutting edge: TGF-β inhibits TH type 2 development through inhibition of GATA-3 expression. J Immunol 165: 4773–4777
Gorelik L et al. (2002) Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J Exp Med 195: 1499–1505
Fantini MC et al. (2004) Cutting edge: TGF-β induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J Immunol 172: 5149–5153
Fantini MC et al. (2005) Transforming growth factor beta-induced Foxp3+ regulatory T cells suppress TH1-mediated experimental colitis. Gut 55: 671–680
Monteleone G et al. (2001) Blocking Smad7 restores TGF-β1 signaling in chronic inflammatory bowel disease. J Clin Invest 108: 601–609
Peschon JJ et al. (1994) Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med 180: 1955–1960
Puel A et al. (1998) Defective IL7R expression in T−B+NK+ severe combined immunodeficiency. Nat Genet 20: 394–397
Becker TC et al. (2002) Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med 195: 1541–1548
Schluns KS et al. (2002) Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J Immunol 168: 4827–4831
Kennedy MK et al. (2000) Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med 191: 771–780
Cooper MA et al. (2002) In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood 100: 3633–3638
Zeng R et al. (2005) Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med 201: 139–148
Ma HL et al. (2003) IL-21 activates both innate and adaptive immunity to generate potent antitumor responses that require perforin but are independent of IFN-γ. J Immunol 171: 608–615
Ozaki K et al. (2002) A critical role for IL-21 in regulating immunoglobulin production. Science 298: 1630–1634
Schorle H et al. (1991) Development and function of T cells in mice rendered interleukin-2-deficient by gene targeting. Nature 352: 621–624
Malek TR (2003) The main function of IL-2 is to promote the development of T regulatory cells. J Leukoc Biol 74: 961–965
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Fantini, M., Becker, C., Kiesslich, R. et al. Drug Insight: novel small molecules and drugs for immunosuppression. Nat Rev Gastroenterol Hepatol 3, 633–644 (2006). https://doi.org/10.1038/ncpgasthep0611
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpgasthep0611
This article is cited by
-
Optimization of FK-506 production in Streptomyces tsukubaensis by modulation of Crp-mediated regulation
Applied Microbiology and Biotechnology (2023)