Abstract
Zeolites and amorphous silica-alumina (ASA), which both provide Brønsted acid sites (BASs), are the most extensively used solid acid catalysts in the chemical industry. It is widely believed that BASs consist only of tetra-coordinated aluminum sites (AlIV) with bridging OH groups in zeolites or nearby silanols on ASA surfaces. Here we report the direct observation in ASA of a new type of BAS based on penta-coordinated aluminum species (AlV) by 27Al-{1H} dipolar-mediated correlation two-dimensional NMR experiments at high magnetic field under magic-angle spinning. Both BAS-AlIV and -AlV show a similar acidity to protonate probe molecular ammonia. The quantitative evaluation of 1H and 27Al sites demonstrates that BAS-AlV co-exists with BAS-AlIV rather than replaces it, which opens new avenues for strongly enhancing the acidity of these popular solid acids.
Similar content being viewed by others
Introduction
The need for efficient and environmentally benign chemical processes has forced the replacement of harmful and corrosive liquid acids by solid acids in various fields of catalysis, including fine chemistry1,2,3, renewable energy production4,5,6, oil refining and petrochemical industries7,8. Silicon- and aluminum-based mixed oxides provide moderate and strong Brønsted acidity and are among the most popular solid acids used in current chemical processes7,9. Briefly, the solid acid catalysts can protonate hydrocarbon molecules to form carbocations and drive important reactions, such as cracking, hydrocracking, isomerization, alkylation and aromatization10,11,12,13,14, through surface complexes or transition states15.
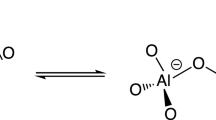
Crystalline zeolites and amorphous silica-alumina (ASA) are two main types of solid acids that contain Brønsted acid sites (BASs). It has been widely believed that only tetra-coordinated aluminum (AlIV) atoms are able to contribute to the formation of BASs in nature16. In crystalline zeolites, the BASs are formed by protons, which compensate the negatively charged oxygens induced by the substitution of Si atoms by AlIV in the framework. The structure of these sites is well known as the bridging Si(OH)AlIV model (Fig. 1a)15,16,17. Replacing Si atoms by more AlIV species can enhance the density of BASs, but it reduces the mean electronegativity of the framework, which thus leads to a decrease of the overall acid strength of BASs18,19. Similarly, AlIV species incorporated into the amorphous silica network are able to generate BASs on ASA9,20,21. The proximity between AlIV and silanol sites in ASA has recently been observed by nuclear magnetic resonance (NMR) correlation experiments between 29Si and 27Al nuclei, the sensitivity of which was enhanced by dynamic nuclear polarization22. However, the strength of these BASs is generally lower than that on crystalline zeolites7 and thus the presence of bridging OH groups (Fig. 1a) in ASA is still strongly under debate22,23.
(a) BAS consisting of a bridging silanol site bonded to AlIV site (Si(OH)Al) in zeolites15. (b) BAS consisting of the flexible coordination between silanol oxygen and neighbouring AlIV (ref. 24). (c) BAS consisting of PBS interacting with AlIV site27. In that later case, the dotted line does not denote a covalent bond but only the close proximity between O and Al atoms.
A flexible coordination between the AlIV atom and the neighbouring silanol oxygen atom (Fig. 1b)9,21,24,25 or a pseudobridging silanol (PBS) with a nearby Al atom (Fig. 1c)26,27 have been proposed22, to account for the longer Al-O distances (2.94–4.43 Å) in ASA26, with respect to those in the crystalline zeolite framework (1.88–2.0 Å)28. So far, most efforts focus on tuning the concentration of AlIV as the main route to increase the Brønsted acidity on zeolites or silica-alumina29,30,31,32,33. However, AlIV species tend to condense, to form an alumina phase at high Al/Si ratios34,35,36, leading to the decrease of Brønsted acidity. For ASA containing solely BASs based on AlIV species (BAS-AlIV), the maximum Brønsted acidity has been obtained at 30 wt% Al loading37,38. In spite of the different BAS models, only AlIV species have been experimentally confirmed to contribute to the formation of BASs in these catalysts. AlV and AlVI species have been shown to act as Lewis acid sites on ASA and zeolites, but, to the best of our knowledge, no experimental evidence of BASs involving these sites has been reported so far39,40,41,42.
Herein, we provide the direct experimental evidence for a new type of BAS-AlV in ASA by dipolar-mediated heteronuclear multiple quantum correlation (D-HMQC) two-dimensional (2D) NMR experiments, which allow the detection of protons via 27Al nuclei, hereafter noted 27Al-{1H}, hence probing the spatial proximities between different Al species and surface protons43,44,45. These experiments show that ASA can contain a large amount of AlV species located near SiOH groups. The acidity of these surface BAS-AlV sites has been demonstrated in this research by the adsorption of basic ammonia molecules, which react with BAS-AlV to form surface ammonium ions.
Results
Probing the connectivity between AlV species and SiOH groups
The ASAs used in this work (see Supplementary Methods) have been prepared according to a previously described procedure9, which generates ASA nanoparticles with a large amount of AlV species. The ASA powders are designated as SA/X, where X is 10 or 50, indicating the molar fraction of Al in the precursor with respect to the total amount of Al and Si atoms. The obtained ASAs have tunable BAS acidity strengths ranging from moderate (SA/10 has an acidity close to zeolite H-X) to large (SA/30–70 have stronger BASs than zeolites H-Y and ZSM-5), depending on the aluminum content, as confirmed by both 13C magic angle spinning (MAS) NMR investigation with probe molecule acetone and ammonia-temperature program desorption (TPD)9. The ASAs exhibited excellent catalytic performances for the conversion of phenylglyoxal with various alcohols, better than that of dealuminated zeolite Y, which hitherto was considered to be the most active solid acid in phenylglyoxal conversion2.
The formation of BAS requires the aluminum atoms to be close to SiOH groups. Such proximity induces a dipolar coupling between 27Al and 1H nuclei, which can efficiently be probed by D-HMQC NMR 2D experiments based on coherence transfers via the 1H-27Al dipolar couplings46,47. As shown in Fig. 2, the correlation at δ27A=50 p.p.m. and δ1H=1.9 p.p.m. in the 27Al-{1H} D-HMQC 2D spectrum of dehydrated SA/50 indicates a close proximity between AlIV species and the proton of SiOH groups. This correlation is ascribed to the Si-OH···AlIV coordination: the typical BAS-AlIV often described for ASA (Fig. 1c)16. A very weak correlation at δ27Al=10 p.p.m. and δ1H=1.1 p.p.m. is assigned to the non-acidic terminal AlVIOH groups often observed on the surface of silica-alumina or zeolites, whereas the low-field broad hump at ca. 6 p.p.m. in the 1H dimension could be caused by the small fraction of hydrogen-bonded AlOH groups16. Nevertheless, the most intense correlation is observed between AlV species (δ27A=30 p.p.m.) and SiOH protons (δ1H=1.9 p.p.m.), which indicates the close proximity between SiOH groups and AlV species, and the presence of Si-OH···AlV coordination (Fig. 2) in dehydrated SA/50.
AlV-based BASs
In zeolites, the substitution of a framework Si atom by an AlIV one to form one SiOHAl acid site (Fig. 1a) can shift the 1H NMR signal of SiOH from ca. 1.8 to 3.6–5.2 p.p.m. (ref. 16). For these catalysts, BAS could be directly evidenced by the cross-peak in 27Al-{1H} D-HMQC 2D spectrum between AlIV species (δ27Al=60 p.p.m.) and the bridging OH groups (δ1H=4.3 p.p.m.)43. However, previous works have shown that the Al atoms with neighbouring SiOH groups (Fig. 1b,c) do not produce such a shift of the 1H MAS signal of these groups5,6,9,16,20,21. Supplementary Fig. 1a,b show that the 1H signal of SA/10 and SA/50 is centred around 1.9 p.p.m., thus indicating a majority of flexible or PBS coordination rather than zeolitic bridging coordination between SiOH groups and either AlIV or AlV species.
Experiments using probe molecules have confirmed the role of flexible or PBS Si-OH···AlIV coordination as BAS in ASA5,6,9,16,20,21. Similar methods using ammonia probe molecules were applied here to demonstrate the acidity of the Si-OH···AlV coordination observed in dehydrated SA/10 and SA/50 (ref. 16). For these samples loaded with ammonia, the 1H signal of ammonium ions was observed at δ1H=6.7 p.p.m., as shown in Supplementary Fig. 1c,d, and commented in Supplementary Note 1. The formation of these ions shows that ammonia reacts with BAS of SA/10 and SA/50.
27Al-{1H} D-HMQC experiments were also carried out to determine the nature of BAS, which protonate the ammonia molecules. Such a strategy based on 1H-27Al correlations has been applied for [Al]MCM-41 loaded with ammonia. For such catalysts, ammonium ions (δ1H=6.7 p.p.m.) were only coupled to AlIV species (δ27A=56 p.p.m.)44. Hence, there was only evidence for BAS-AlIV on the surface of [Al]MCM-41, which protonated ammonia to ammonium ions. As seen in Fig. 3, a correlation between NH4+ ions (δ1H=6.7 p.p.m.) and AlIV (δ27Al=50 p.p.m.) is also observed in 27Al-{1H} D-HMQC spectra of SA/10 and SA/50, showing that the BAS-AlIV sites are also present on the surface of ASAs (Fig. 4a). Interestingly, these spectra also exhibit cross-peaks between AlV species (δ27Al=30 p.p.m.) and NH4+ ions (δ1H=6.7 p.p.m.) in both SA/10 and SA/50. As seen in Fig. 3e, the intensity of this AlV-NH4+ cross-peak is comparable to that of the AlIV-NH4+ one. Given the BAS density ranging from 0.16 to 0.36 H+ nm−2 in the investigated ASA samples (Supplementary Table 1), each ammonia molecule only interacts with one BAS. The distance between the aluminum atom and the neighbouring silanol oxygen in ASA ranges from ca. 2.94 to 4.43 Å26 and the N–H bond length in ammonia is only 1.02 Å48. As the heteronuclear coherence transfer in 27Al-{1H} D-HMQC is only effective up to a few angstroms, the protons of Si-O−(NH4)+···Al environment only interact with the neighbouring Al. The observation of an AlV-NH4+ cross-peak in Fig. 3 at (30, 6.7) p.p.m. directly confirmed that ammonia is protonated on a BAS containing AlV: the Si-OH···AlV group.
The dehydrated SA/10 (a) and SA/50 (b) samples were loaded with ammonia and evacuated at 373 K for 1 h, and the spectra were recorded at 18.8 T with νR=20 kHz and τrec=900 μs. The 1H slices at the shifts of AlIV and AlV sites of SA/10 extracted from the 2D spectrum (a) are displayed in subfigures (c,d), respectively. The subfigure (e) shows the 27Al slice at the shift of NH4+ protons in SA/50 extracted from the spectrum (b).
The comparison of Supplementary Fig. 1c,d shows that more ammonia molecules are protonated on BAS-AlV in SA/50 than in SA/10. Combined with quantitative 1H NMR investigations (Supplementary Fig. 1) and the quadrupolar parameters (Supplementary Table 2 and Supplementary Note 2) obtained from 27Al one-dimensional MAS (Supplementary Fig. 3) and 2D multiple quantum MAS (Supplementary Fig. 2) NMR experiments, the analysis of 27Al cross-peak intensities in 27Al-{1H} D-HMQC spectra (Supplementary Fig. 4 and Supplementary Note 3) revealed that the population densities of both BAS-AlIV and -AlV on SA/50 (0.078 and 0.053 mmol g−1) were both higher than those of SA/10 (0.058 and 0.039 mmol g−1). This result suggests that BAS-AlIV and -AlV can co-exist on the surface rather than replacing each other and the population of both acid sites can be amplified by increasing Al content. Thus, this observation is promising for enhancing the population of BAS on ASA without limitation imposed by the Al contents. It should be noted that the F2 projections of 27Al-{1H} D-HMQC 2D spectra are almost identical for dehydrated and ammonia-loaded SA/50 (see Supplementary Fig. 5 and Supplementary Note 4). Therefore, Si-OH···AlV and Si-OH···AlIV coordinations remained unchanged after the protonation of ammonia (as shown in Fig. 4). No Si-OH···AlIV have been transferred to Si-OH-AlV permanently after the adsorption of ammonia. In other words, the NMR results do not show the formation of permanent covalent bridges between silicate and AlIV or AlV sites in ASA samples after the deprotonation of BAS reacting with ammonia. Ammonia partially interacting with surface AlIV or AlV sites (Fig. 3b and Supplementary Fig. 6) was also observed, which has been assigned to ammonia adsorbed on Lewis sites (Supplementary Note 5).
As shown in Fig. 1, a surface bridging SiOHAl (Fig. 1a), a flexible coordination of SiOH and Al (Fig. 1b), or a pseudo-bridge between SiOH and Al atom (Fig. 1c) have been proposed for the formation of BAS-AlIV on ASAs. By analogy, similar structures might also contribute to the formation of BAS-AlV. The PBS model permits an explanation of the observation of the 1H NMR signal of SiOH at 1.9 p.p.m. in both Fig. 2 and Supplementary Fig. 1, whereas this 1H signal of bridging OH groups (Fig. 1a) should occur at 3.6–5.2 p.p.m. If bridging OH groups are present in the investigated ASAs, their concentration must be below the limit of detection of the one-dimensional NMR MAS spectra of Supplementary Fig. 1. Nevertheless, the current NMR data cannot rule out, in addition to PBS, the presence of bridging silanol groups in low concentration in ASA samples. These elusive strong BASs may also contribute to the catalytic activity in spite of their low concentration. The identification of all catalytic BASs in ASAs is beyond the scope of the present study, which is mainly to report the existence of BASs based on AlV environments. A final assessment of the local structure of BAS-AlV will require further experimental and theoretical work.
In summary, a new type of BAS-AlV has been directly observed by 27Al-{1H} D-HMQC NMR spectroscopy. Hitherto, it was widely accepted that AlV sites only provide Lewis acidity39,40,41,42, and that solely AlIV ones contribute to the formation of BASs in aluminosilicate. However, we prove here by NMR experiments that similar to AlIV sites, AlV ones interact with neighbouring SiOH groups in ASA and behave as BASs in agreement with the PBS model. BAS-AlIV and -AlV seem to be structurally similar and show comparable acidity to protonate ammonia. Finally, a very important implication emerging from this work is that both AlIV and AlV species can co-exist on the surface of ASA. This feature facilitates that the total population density of BAS can be increased up to 70% by increasing the Al content, an amount much higher than the maximum Al loading of ca. 30% at which maximum acidity on ASA containing exclusively BAS-AlIV is achieved37,38. Hence, our findings not only report the existence of a new type of BAS in nature, but also open new avenues for creating high-performance solid acid catalysts containing AlV species, which will be promising for sustainable oil-refining and many industrial chemical processes.
Methods
27Al-{1H} D-HMQC 2D experiment
All NMR experiments were recorded on a Bruker Avance III 18.8 T (1H Larmor frequency of 800 MHz) spectrometer equipped with a 3.2 mm double-resonance MAS probe, in which rotors were spun at νR=20 kHz. In the D-HMQC sequence, we have detected the 27Al nuclei to benefit from their fast longitudinal relaxation times and the 1H-27Al dipolar couplings were reintroduced by applying a SR recoupling on the 1H channel49. The 1H radiofrequency amplitudes for the 90° pulses and the SR
recoupling on the 1H channel49. The 1H radiofrequency amplitudes for the 90° pulses and the SR recoupling were equal to ν1=62.5 and 40 kHz, respectively. The central transition selective pulse lengths on 27Al were 8 and 16 μs for 90° and 180° pulses, respectively, that is, radiofrequency field amplitude ν1=10 kHz. The total dipolar recoupling time, τrec, ranged from 700 to 1,000 μs depending on the sample. The 2D spectra resulted from the accumulation of 512 transients for each of 20 t1 increments with Δt1=50 μs and a recycle delay=1 s, that is, a total experiment time of about 3 h. Additional details about NMR experiments are given in the Supplementary Methods.
recoupling were equal to ν1=62.5 and 40 kHz, respectively. The central transition selective pulse lengths on 27Al were 8 and 16 μs for 90° and 180° pulses, respectively, that is, radiofrequency field amplitude ν1=10 kHz. The total dipolar recoupling time, τrec, ranged from 700 to 1,000 μs depending on the sample. The 2D spectra resulted from the accumulation of 512 transients for each of 20 t1 increments with Δt1=50 μs and a recycle delay=1 s, that is, a total experiment time of about 3 h. Additional details about NMR experiments are given in the Supplementary Methods.
Data availability
The data that support the findings of this study are available upon request from the corresponding author J.H. and J.-P.A.
Additional information
How to cite this article: Wang, Z. et al. Brønsted acid sites based on penta-coordinated aluminum species. Nat. Commun. 7, 13820 doi: 10.1038/ncomms13820 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Corma, A., Iborra, S. & Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 107, 2411–2502 (2007).
Wang, Z., Jiang, Y., Baiker, A. & Huang, J. Efficient acid-catalyzed conversion of phenylglyoxal to mandelates on flame-derived silica/alumina. ACS Catal 3, 1573–1577 (2013).
Wang, Z., Jiang, Y., Hunger, M., Baiker, A. & Huang, J. Catalytic performance of Brønsted and Lewis acid sites in phenylglyoxal conversion on flame-derived silica-zirconia. ChemCatChem 6, 2970–2975 (2014).
Huber, G. W., Iborra, S. & Corma, A. Synthesis of transportation fuels from biomass: chemistry, catalysts, and engineering. Chem. Rev. 106, 4044–4098 (2006).
Huang, J., Jiang, Y., van Vegten, N., Hunger, M. & Baiker, A. Tuning the support acidity of flame-made Pd/SiO2-Al2O3 catalysts for chemoselective hydrogenation. J. Catal. 281, 352–360 (2011).
Wang, Z. et al. Palladium-doped silica–alumina catalysts obtained from double-flame FSP for chemoselective hydrogenation of the model aromatic ketone acetophenone. J. Catal. 302, 10–19 (2013).
Corma, A. Inorganic solid acids and their use in acid-catalyzed hydrocarbon reactions. Chem. Rev. 95, 559–614 (1995).
Busca, G. Acid catalysts in industrial hydrocarbon chemistry. Chem. Rev. 107, 5366–5410 (2007).
Huang, J., van Vegten, N., Jiang, Y., Hunger, M. & Baiker, A. Increasing the Brønsted acidity of flame-derived silica/alumina up to zeolitic strength. Angew. Chem. Int. Ed. 49, 7776–7781 (2010).
Engelhardt, J. & Hall, W. K. Peculiarities observed in H-D exchange between perdeuterioisobutane and H-zeolites. J. Catal. 151, 1–9 (1995).
Hua, W. et al. H/D exchange reaction between isobutane and acidic USY zeolite: a mechanistic study by mass spectrometry and in situ NMR. J. Catal. 204, 460–465 (2001).
Olah, G. A. Carbocations and electrophilic reactions. Angew. Chem. Int. Ed. 12, 173–212 (1973).
Olah, G. A. Friedel-Crafts Chemistry Wiley-Interscience (1973).
Olah, G. A. My search for carbocations and their role in chemistry (Nobel lecture). Angew. Chem. Int. Ed. 34, 1393–1405 (1995).
Weitkamp, J. & Hunger, M. in Studies in Surface Science and Catalysis eds Ejka J., van Bekkum H., Corma A., Schüth F. 787–835Elsevier (2007).
Jiang, Y., Huang, J., Dai, W. & Hunger, M. Solid-state nuclear magnetic resonance investigations of the nature, property, and activity of acid sites on solid catalysts. Solid State Nucl. Magn. Reson. 39, 116–141 (2011).
Li, S. et al. Brønsted/Lewis acid synergy in dealuminated HY zeolite: a combined solid-state NMR and theoretical calculation study. J. Am. Chem. Soc. 129, 11161–11171 (2007).
Jacobs, P. A., Mortier, W. J. & Uytterhoeven, J. B. Properties of zeolites in relation to their electronegativity - acidity, carboniogenic activity and strength of interaction in transition-metal complexes. J. Inorg. Nucl. Chem. 40, 1919–1923 (1978).
Mortier, W. J. Zeolite electronegativity related to physicochemical properties. J. Catal. 55, 138–145 (1978).
Wang, Z. et al. One-step room-temperature synthesis of Al MCM-41 materials for the catalytic conversion of phenylglyoxal to ethylmandelate. ChemCatChem 5, 3889–3896 (2013).
Luo, Q. et al. Using trimethylphosphine as a probe molecule to study the acid states in Al-MCM-41 materials by solid-state NMR spectroscopy. J. Phys. Chem. B 107, 2435–2442 (2003).
Valla, M. et al. Atomic description of the interface between silica and alumina in aluminosilicates through dynamic nuclear polarization surface-enhanced nmr spectroscopy and first-principles calculations. J. Am. Chem. Soc. 137, 10710–10719 (2015).
Hensen, E. J. M. et al. Acidity characterization of amorphous silica-alumina. J. Phys. Chem. C 116, 21416–21429 (2012).
Omegna, A., van Bokhoven, J. A. & Prins, R. Flexible aluminum coordination in alumino-silicates. Structure of zeolite H-USY and amorphous silica-alumina. J. Phys. Chem. B 107, 8854–8860 (2003).
Hunger, M., Schenk, U., Breuninger, M., Glaser, R. & Weitkamp, J. Characterization of the acid sites in MCM-41-type materials by spectroscopic and catalytic techniques. Micro. Meso. Mater. 27, 261–271 (1999).
Chizallet, C. & Raybaud, P. Pseudo-bridging silanols as versatile Brønsted acid sites of amorphous aluminosilicate surfaces. Angew. Chem. Int. Ed. 48, 2891–2893 (2009).
Chizallet, C. & Raybaud, P. Acidity of Amorphous silica-alumina: from coordination promotion of Lewis sites to proton transfer. ChemPhysChem 11, 105–108 (2010).
Eichler, U., Brandle, M. & Sauer, J. Predicting absolute and site specific acidities for zeolite catalysts by a combined quantum mechanics interatomic potential function approach. J. Phys. Chem. B 101, 10035–10050 (1997).
Dewitte, B. M., Grobet, P. J. & Uytterhoeven, J. B. Pentacoordinated aluminum in noncalcined amorphous aluminosilicates, prepared in alkaline and acid-medium. J. Phys. Chem. 99, 6961–6965 (1995).
Williams, M. F. et al. Hydrogenation of tetralin on silica-alumina-supported Pt catalysts I. Physicochemical characterization of the catalytic materials. J. Catal. 251, 485–496 (2007).
de Boer, J. H. Constitution and properties of silica-alumina-catalysts. Discuss. Farad. Soc. 52, 109–112 (1971).
Haag, W. O., Lago, R. M. & Weisz, P. B. The active site of acidic aluminosilicate catalysts. Nature 309, 589–591 (1984).
Xu, B. et al. Strong Brønsted acidity in amorphous silica−aluminas. J. Phys. Chem. C 111, 12075–12079 (2007).
Matsumoto, A., Chen, H., Tsutsumi, K., Grun, M. & Unger, K. Novel route in the synthesis of MCM-41 containing framework aluminum and its characterization. Micro. Meso. Mater. 32, 55–62 (1999).
Eimer, G. A., Pierella, L. B., Monti, G. A. & Anunziata, O. A. Synthesis and characterization of Al-MCM-41 and Al-MCM-48 mesoporous materials. Catal. Lett. 78, 65–75 (2002).
Hensen, E. et al. Formation of acid sites in amorphous silica-alumina. J. Catal. 269, 201–218 (2010).
Tanabe, K. Solid Acids and Bases and Their Catalytic Properties Academic Press (1970).
Hunger, M. et al. High-resolution proton magnetic-resonance and catalytic studies concerning Brønsted centers of amorphous Al2O3-SiO2 solids. Chem. Phys. Lett. 100, 29–33 (1983).
Coster, D., Blumenfeld, A. L. & Fripiat, J. J. Lewis acid sites and surface aluminum in aluminas and zeolites: a high-resolution NMR study. J. Phys. Chem. 98, 6201–6211 (1994).
Ma, D. et al. An investigation of the roles of surface aluminum and acid sites in the zeolite MCM-22. Chem. Eur. J. 8, 162–170 (2002).
Borade, R., Adnot, A. & Kaliaguine, S. An XPS study of acid sites in dehydroxylated Y zeolites. J. Mol. Catal. 61, L7–L14 (1990).
Kwak, J. H., Mei, D., Peden, C. H. F., Rousseau, R. & Szanyi, J. (100) facets of gamma-Al2O3: the active surfaces for alcohol dehydration reactions. Catal. Lett. 141, 649–655 (2011).
Li, S. et al. Extra-framework aluminium species in hydrated faujasite zeolite as investigated by two-dimensional solid-state NMR spectroscopy and theoretical calculations. Phys. Chem. Chem. Phys. 12, 3895–3903 (2010).
Janicke, M. T. et al. Aluminum incorporation and interfacial structures in MCM-41 mesoporous molecular sieves. J. Am. Chem. Soc. 120, 6940–6951 (1998).
Wang, Y. Y., Mu, Y., Sun, Y. J. & Li, J. Y. Two new four-connected zeolite-like magnesium aluminophosphates with intersecting 8-ring channels. RSC Adv. 4, 56288–56293 (2014).
Lafon, O. et al. Indirect detection via spin-1/2 nuclei in solid state NMR spectroscopy: application to the observation of proximities between protons and quadrupolar nuclei. J. Phys. Chem. A. 113, 12864–12878 (2009).
Tricot, G. et al. Structural characterisation of phosphate materials: new insights into the spatial proximities between phosphorus and quadrupolar nuclei using the D-HMQC MAS NMR technique. Phys. Chem. Chem. Phys. 13, 16786–16794 (2011).
Haynes, W. M. in Handbook of Chemistry and Physics (ed. Ohio) 96, CRC Press (2015).
Brinkmann, A. & Kentgens, A. P. M. Proton-selective 17O−1H distance measurements in fast magic-angle-spinning solid-state NMR spectroscopy for the determination of hydrogen bond lengths. J. Am. Chem. Soc. 128, 14758–14759 (2006).
Acknowledgements
J.H. and C.S. acknowledge the financial supports from Australian Research Council Discovery Projects (DP150103842). J.H. thanks Faculty’s Energy and Materials Clusters and MCR scheme, and the International Project Development Funding at the University of Sydney. O.L., J.L. and J.-P.A. are grateful for funding provided by the Region Nord/Pas de Calais (France), Europe (FEDER), CNRS, Ministère de l’Enseignement Supérieur et de la Recherche, CPER, Chevreul Institute (FR 2638), Infrastructure de Recherche en Eésonance Magnétique Nucléaire à Très Haut Champ (IR-RMN, FR 3050), ENSCL, the University of Lille and contract ANR-14-CE07-0009.
Author information
Authors and Affiliations
Contributions
J.H., Y.J. and C.S. designed the study. Y.J. and A.B. prepared the samples. Z.W., O.L., J.T. and J.H. performed the NMR experiments and structural assignation. J.H. and J.-P.A. supervised the scientific work. J.H. and Z.W. contributed to writing the paper, and O.L., J.-P.A., A.B. and C.S. revised it.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures, Supplementary Tables, Supplementary Methods, Supplementary Notes and Supplementary References. (PDF 1264 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wang, Z., Jiang, Y., Lafon, O. et al. Brønsted acid sites based on penta-coordinated aluminum species. Nat Commun 7, 13820 (2016). https://doi.org/10.1038/ncomms13820
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms13820
This article is cited by
-
Acidity enhancement through synergy of penta- and tetra-coordinated aluminum species in amorphous silica networks
Nature Communications (2020)
-
Dehydroaromatization of methane over Mo/ZSM-5 zeolites: influence of aluminum distribution in the crystals
Reaction Kinetics, Mechanisms and Catalysis (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.