Abstract
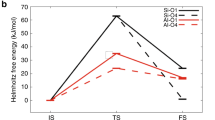
Steric confinement in zeolites influences the catalytic conversion of alkanes. For zeolitic Brønsted acid sites, proximate extra-framework species introduce additional confinement to the pore constraints, enhancing the catalytic activity of alkane cracking. Although extra-framework alumina has been the most studied, here we report the element-specific impact of silica species. By grafting extra-framework silica species close to Brønsted acid sites in H-ZSM-5 zeolite, the binding of bases like pyridine and amines is strengthened via van der Waals interactions with their aryl or alkyl chains. Brønsted acid sites close to extra-framework silica achieve a higher reaction rate of protolytic cracking of n-pentane via enthalpic (unlike entropic, as with extra-framework alumina) stabilization of the transition state by 24–51 kJ mol−1. The lower activation energy points to an earlier transition state than in the presence of extra-framework alumina, with a better stabilization of the carbonium ions in the transition state compared to the parent zeolite.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data are available from the corresponding authors upon reasonable request.
References
Rahimi, N. & Karimzadeh, R. Catalytic cracking of hydrocarbons over modified ZSM-5 zeolites to produce light olefins: a review. Appl. Catal. A 398, 1–17 (2011).
Babitz, S. M. M. et al. Monomolecular cracking of n-hexane on Y, MOR, and ZSM-5 zeolites. Appl. Catal. A 179, 71–86 (1999).
Weitkamp, J., Jacobs, P. A. & Martens, J. A. Isomerization and hydrocracking of C9 through C16 n-alkanes on Pt/HZSM-5 zeolite. Appl. Catal. 8, 123–141 (1983).
Young, L. B., Butter, S. A. & Kaeding, W. W. Shape selective reactions with zeolite catalysts: III. Selectivity in xylene isomerization, toluene-methanol alkylation, and toluene disproportionation over ZSM-5 zeolite catalysts. J. Catal. 76, 418–432 (1982).
Xu, B., Sievers, C., Hong, S. B., Prins, R. & van Bokhoven, J. A. Catalytic activity of Brønsted acid sites in zeolites: intrinsic activity, rate-limiting step, and influence of the local structure of the acid sites. J. Catal. 244, 163–168 (2006).
Ward, J. W. The nature of active sites on zeolites: V. In situ spectroscopic observations of hydrogen Y zeolite during cumene cracking. J. Catal. 11, 259–260 (1968).
Kissin, Y. V. Chemical mechanisms of catalytic cracking over solid acidic catalysts: alkanes and alkenes. Catal. Rev. Sci. Eng. 43, 85–146 (2001).
Ozaki, A. & Kimura, K. The effective site on acid catalysts revealed in n-butene isomerization. J. Catal. 3, 395–405 (1964).
Asuquo, R. A., Edermirth, G. & Lercher, J. A. n-Butane isomerization over acidic mordenite. J. Catal. 155, 376–382 (1995).
Feller, A., Guzman, A., Zuazo, I. & Lercher, J. A. On the mechanism of catalyzed isobutane/butene alkylation by zeolites. J. Catal. 224, 80–93 (2004).
Naccache, C., Ren, C. F. & Coudurier, G. Strength of acid sites in zeolites using UV-visible spectroscopy: effect of Al content. Stud. Surf. Sci. Catal. 49, 661–668 (1989).
Masuda, T., Fujikata, Y., Mukai, S. R. & Hashimoto, K. Changes in catalytic activity of MFI-type zeolites caused by dealumination in a steam atmosphere. Appl. Catal. A 172, 73–83 (1998).
Li, S. et al. Brønsted/Lewis acid synergy in dealuminated HY zeolite: a combined solid-state NMR and theoretical calculation study. J. Am. Chem. Soc. 129, 11161–11171 (2007).
Chu, C. T. W. & Chang, C. D. Isomorphous substitution in zeolite frameworks. 1. Acidity of surface hydroxyls in [B]-, [Fe]-, [Ga]-, and [Al]-ZSM-5. J. Phys. Chem. 89, 1569–1571 (1985).
Losch, P. et al. Proton mobility, intrinsic acid strength, and acid site location in zeolites revealed by varying temperature infrared spectroscopy and density functional theory studies. J. Am. Chem. Soc. 140, 17790–17799 (2018).
Jones, A. J., Carr, R. T., Zones, S. I. & Iglesia, E. Acid strength and solvation in catalysis by MFI zeolites and effects of the identity, concentration and location of framework heteroatoms. J. Catal. 312, 58–68 (2014).
Ward, J. W. The nature of active sites on zeolites. VIII. Rare earth Y zeolite. J. Catal. 13, 321–327 (1969).
Xiaoning, W. et al. Effects of light rare earth on acidity and catalytic performance of HZSM-5 zeolite for catalytic cracking of butane to light olefins. J. Rare Earths 25, 321–328 (2007).
Martins, A., Silva, J. M. & Ribeiro, M. F. Influence of rare earth elements on the acid and metal sites of Pt/HBEA catalyst for short chain n-alkane hydroisomerization. Appl. Catal. A 466, 293–299 (2013).
Sastre, G. & Corma, A. The confinement effect in zeolites. J. Mol. Catal. A 305, 3–7 (2009).
Gounder, R. & Iglesia, E. The catalytic diversity of zeolites: confinement and solvation effects within voids of molecular dimensions. Chem. Commun. 49, 3491–3509 (2013).
Eder, F. & Lercher, J. A. On the role of the pore size and tortuosity for sorption of alkanes in molecular sieves. J. Phys. Chem. B 101, 1273–1278 (1997).
De Moor, B. A., Reyniers, M.-F., Gobin, O. C., Lercher, J. A. & Marin, G. B. Adsorption of C2–C8 n-alkanes in zeolites. J. Phys. Chem. C 115, 1204–1219 (2010).
Janda, A., Vlaisavljevich, B., Lin, L. C., Smit, B. & Bell, A. T. Effects of zeolite structural confinement on adsorption thermodynamics and reaction kinetics for monomolecular cracking and dehydrogenation of n-butane. J. Am. Chem. Soc. 138, 4739–4756 (2016).
Boronat, M. & Corma, A. What is measured when measuring acidity in zeolites with probe molecules? ACS Catal. https://doi.org/10.1021/acscatal.8b04317 (2019).
Wang, Q., Cui, Z. M., Cao, C. Y. & Song, W. G. 0.3 Å makes the difference: dramatic changes in methanol-to-olefin activities between H-ZSM-12 and H-ZSM-22 zeolites. J. Phys. Chem. C 115, 24987–24992 (2011).
Silaghi, M.-C., Chizallet, C. & Raybaud, P. Challenges on molecular aspects of dealumination and desilication of zeolites. Microporous Mesoporous Mater. 191, 82–96 (2014).
Silaghi, M.-C., Chizallet, C., Sauer, J. & Raybaud, P. Dealumination mechanisms of zeolites and extra-framework aluminum confinement. J. Catal. 339, 242–255 (2016).
Schallmoser, S. et al. Impact of the local environment of Brønsted acid sites in ZSM-5 on the catalytic activity in n-pentane cracking. J. Catal. 316, 93–102 (2014).
Zhang, Y. et al. Promotion of protolytic pentane conversion on H-MFI zeolite by proximity of extra-framework aluminum oxide and Brønsted acid sites. J. Catal. 370, 424–433 (2019).
Lago, R. M. et al. The nature of the catalytic sites in HZSM-5-activity enhancement. Stud. Surf. Sci. Catal. 28, 677–684 (1986).
van Bokhoven, J. A. et al. An explanation for the enhanced activity for light alkane conversion in mildly steam dealuminated mordenite: the dominant role of adsorption. J. Catal. 202, 129–140 (2001).
Van Bokhoven, J. A. et al. Observation of a compensation relation for monomolecular alkane cracking by zeolites: the dominant role of reactant sorption. J. Catal. 224, 50–59 (2004).
Yu, Z., Wang, Q., Chen, L. & Deng, F. Brønsted/Lewis acid sites synergy in H-MCM-22 zeolite studied by 1H and 27Al DQ-MAS NMR spectroscopy. Chin. J. Catal. 33, 129–139 (2012).
Haag, W. O. Catalysis by zeolites—science and technology. Stud. Surf. Sci. Catal. 84, 1375–1394 (1994).
Li, S. et al. Probing the spatial proximities among acid sites in dealuminated H-Y zeolite by solid-state NMR spectroscopy. J. Phys. Chem. C 112, 14486–14494 (2008).
Gounder, R., Jones, A. J., Carr, R. T. & Iglesia, E. Solvation and acid strength effects on catalysis by faujasite zeolites. J. Catal. 286, 214–223 (2012).
Xue, N. et al. Hydrolysis of zeolite framework aluminum and its impact on acid catalyzed alkane reactions. J. Catal. 365, 359–366 (2018).
Zheng, J. et al. A periodic DFT study of the synergistic mechanisms between extraframework aluminum species and Brønsted acid sites in HY zeolites. Ind. Eng. Chem. Res. 59, 2736–2744 (2020).
O’Connor, C. T., Möller, K. P. & Manstein, H. The effect of silanisation on the catalytic and sorption properties of zeolites. KONA Powder Part. J. 25, 230–236 (2007).
Niwa, M., Kato, M., Hattori, T. & Murakami, Y. Fine control of the pore-opening size of zeolite ZSM-5 by chemical vapor deposition of silicon methoxide. J. Phys. Chem. 90, 6233–6237 (1986).
Zheng, S., Heydenrych, H. R., Röger, H. P., Jentys, A. & Lercher, J. A. On the enhanced selectivity of HZSM-5 modified by chemical liquid deposition. Top. Catal. 22, 101–106 (2003).
Janda, A. & Bell, A. T. Effects of Si/Al ratio on the distribution of framework Al and on the rates of alkane monomolecular cracking and dehydrogenation in H-MFI. J. Am. Chem. Soc. 135, 19193–19207 (2013).
Parrillo, D. J., Adamo, A. T., Kokotailo, G. T. & Gorte, R. J. Amine adsorption in H-ZSM-5. Appl. Catal. 67, 107–118 (1990).
Ledoux, R. L. & White, J. L. Infrared studies of hydrogen bonding interaction between kaolinite surfaces and intercalated potassium acetate, hydrazine, formamide, and urea. J. Colloid Interface Sci. 21, 127–152 (1966).
Vansant, E. F., Van Der Voort, P. & Vrancken, K. C. Characterization and Chemical Modification of the Silica Surface Vol. 93, 2960–2970 (Elsevier, 1995).
Zecchina, A. et al. Low-temperature Fourier-transform infrared investigation of the interaction of CO with nanosized ZSM5 and silicalite. J. Chem. Soc. Faraday Trans. 88, 2959–2969 (1992).
Trombetta, M., Armaroli, T., Gutièrrez Alejandre, A., Ramirez Solis, J. & Busca, G. An FT-IR study of the internal and external surfaces of HZSM5 zeolite. Appl. Catal. A 192, 125–136 (2000).
Guisnet, M., Ayrault, P., Coutanceau, C., Alvarez, M. F. & Datka, J. Acid properties of dealuminated beta zeolites studied by IR spectroscopy. J. Chem. Soc. Faraday Trans. 93, 1661–1665 (1997).
Zholobenko, V. L., Kustov, L. M., Borovkov, V. Y. & Kazansky, V. B. A new type of acidic hydroxyl groups in ZSM-5 zeolite and in mordenite according to diffuse reflectance IR spectroscopy. Zeolites 8, 175–178 (1988).
Jentys, A., Warecka, G., Derewinski, M. & Lercher, J. A. Adsorption of water on ZSM 5 zeolites. J. Phys. Chem. 93, 4837–4843 (1989).
Loeffler, E. et al. Study of different states of nonframework aluminum in hydrothermally dealuminated HZSM-5 zeolites using diffuse reflectance IR spectroscopy. Zeolites 10, 266–271 (1990).
Kung, M. C. & Kung, H. H. IR studies of NH3, pyridine, CO, and NO adsorbed on transition metal oxides. Catal. Rev. 27, 425–460 (1985).
Chakraborty, B. & Viswanathan, B. Surface acidity of MCM-41 by in situ IR studies of pyridine adsorption. Catal. Today 49, 253–260 (1999).
Emeis, C. A. Determination of integrated molar extinction coefficients for infrared absorption bands of pyridine adsorbed on solid acid catalysts. J. Catal. 141, 347–354 (1993).
Eder, F., Stockenhuber, M. & Lercher, J. A. Brønsted acid site and pore controlled siting of alkane sorption in acidic molecular sieves. J. Phys. Chem. B 101, 5414–5419 (1997).
Kotrel, S., Knözinger, H. & Gates, B. C. The Haag–Dessau mechanism of protolytic cracking of alkanes. Microporous Mesoporous Mater. 35, 11–20 (2000).
Al‐majnouni, K. A., Yun, J. H. & Lobo, R. F. High‐temperature produced catalytic sites selective for n‐alkane dehydrogenation in acid zeolites: the case of HZSM‐5. ChemCatChem 3, 1333–1341 (2011).
Liu, Y. et al. Solvent-determined mechanistic pathways in zeolite-H-BEA-catalysed phenol alkylation. Nat. Catal. 1, 141–147 (2018).
Janda, A., Vlaisavljevich, B., Lin, L.-C., Smit, B. & Bell, A. T. Effects of zeolite structural confinement on adsorption thermodynamics and reaction kinetics for monomolecular cracking and dehydrogenation of n-butane. J. Am. Chem. Soc. 138, 4739–4756 (2016).
Jentys, A., Kleestorfer, K. & Vinek, H. Concentration of surface hydroxyl groups on MCM-41. Microporous Mesoporous Mater. 27, 321–328 (1999).
Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2, 73–78 (2012).
Russo, T. V., Martin, R. L. & Hay, P. J. Density functional calculations on first‐row transition metals. J. Chem. Phys. 101, 7729–7737 (1994).
Tao, J., Perdew, J. P., Tang, H. & Shahi, C. Origin of the size-dependence of the equilibrium van der Waals binding between nanostructures. J. Chem. Phys. 148, 074110 (2018).
Miehlich, B., Savin, A., Stoll, H. & Preuss, H. Results obtained with the correlation energy density functionals of Becke and Lee, Yang and Parr. Chem. Phys. Lett. 157, 200–206 (1989).
Adamo, C. & Barone, V. Toward reliable density functional methods without adjustable parameters: the PBE0 model. J. Chem. Phys. 110, 6158–6170 (1999).
Schäfer, A., Horn, H. & Ahlrichs, R. Fully optimized contracted Gaussian basis sets for atoms Li to Kr. J. Chem. Phys. 97, 2571–2577 (1992).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005).
Neese, F. An improvement of the resolution of the identity approximation for the formation of the Coulomb matrix. J. Comput. Chem. 24, 1740–1747 (2003).
Vahtras, O., Almlöf, J. & Feyereisen, M. W. Integral approximations for LCAO-SCF calculations. Chem. Phys. Lett. 213, 514–518 (1993).
Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 8, 1057–1065 (2006).
Neese, F., Wennmohs, F., Hansen, A. & Becker, U. Efficient, approximate and parallel Hartree–Fock and hybrid DFT calculations. A ‘chain-of-spheres’ algorithm for the Hartree–Fock exchange. Chem. Phys. 356, 98–109 (2009).
Ehrlich, S., Moellmann, J., Reckien, W., Bredow, T. & Grimme, S. System‐dependent dispersion coefficients for the DFT‐D3 treatment of adsorption processes on ionic surfaces. ChemPhysChem 12, 3414–3420 (2011).
Baerlocher, C. & McCusker, L. B. Database of Zeolite Structures (International Zeolite Association Structure Commission, accessed on 09 June 2020); http://www.iza-structure.org/databases/
Acknowledgements
Conceptual work was supported by the US Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences (BES), Division of Chemical Sciences, Geosciences and Biosciences (Impact of catalytically active centers and their environment on rates and thermodynamic states along reaction paths, FWP 47319). We gratefully acknowledge the Leibniz Supercomputing Center for funding this project by providing computing time on their Linux-Cluster. R.Z. is grateful to the Chinese Scholarship Council for financial support. We also acknowledge M. Iqbal and M. Neukamm for technical support concerning the nitrogen physisorption and elemental analysis, respectively.
Funding
Open Access funding enabled and organized by Projekt DEAL
Author information
Authors and Affiliations
Contributions
R.Z. carried out experimental preparation of the catalyst samples, their reactions and characterization. R.K. performed the theoretical calculations. Y.Z., M.S.-S. and R.B.-D. cooperated with the discussion and provided valuable suggestions. Y.L. and J.A.L. supervised the work and provided guidance throughout the project. All authors have given their approval to the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Xiaolei Fan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–19, Tables 1–7, Notes 1–3 and refs. 1–4.
Supplementary Data 1
MFI cluster structure.
Supplementary Data 2
Sample Orca input file used for geometry optimizations.
Supplementary Data 3
Sample Orca input file used for electronic property calculations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, R., Khare, R., Zhang, Y. et al. Promotion of adsorptive and catalytic properties of zeolitic Brønsted acid sites by proximal extra-framework Si(OH)x groups. Nat Catal 6, 68–79 (2023). https://doi.org/10.1038/s41929-022-00906-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-022-00906-z