Abstract
Mitotic spindle formation relies on the stochastic capture of microtubules at kinetochores. Kinetochore architecture affects the efficiency and fidelity of this process with large kinetochores expected to accelerate assembly at the expense of accuracy, and smaller kinetochores to suppress errors at the expense of efficiency. We demonstrate that on mitotic entry, kinetochores in cultured human cells form large crescents that subsequently compact into discrete structures on opposite sides of the centromere. This compaction occurs only after the formation of end-on microtubule attachments. Live-cell microscopy reveals that centromere rotation mediated by lateral kinetochore–microtubule interactions precedes the formation of end-on attachments and kinetochore compaction. Computational analyses of kinetochore expansion–compaction in the context of lateral interactions correctly predict experimentally observed spindle assembly times with reasonable error rates. The computational model suggests that larger kinetochores reduce both errors and assembly times, which can explain the robustness of spindle assembly and the functional significance of enlarged kinetochores.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
McIntosh, J. R., Molodtsov, M. I. & Ataullakhanov, F. I. Biophysics of mitosis. Q. Rev. Biophys. 45, 147–207 (2012).
Walczak, C. E., Cai, S. & Khodjakov, A. Mechanisms of chromosome behaviour during mitosis. Nat. Rev. Mol. Cell Biol. 11, 91–102 (2010).
Hayden, J. H., Bowser, S. S. & Rieder, C. L. Kinetochores capture astral microtubules during chromosome attachment to the mitotic spindle: direct visualization in live newt lung cells. J. Cell Biol. 111, 1039–1045 (1990).
Tanaka, K. et al. Molecular mechanisms of kinetochore capture by spindle microtubules. Nature 434, 987–994 (2005).
Kirschner, M. & Mitchison, T. Beyond self-assembly: from microtubules to morphogenesis. Cell 45, 329–342 (1986).
Wan, X. et al. Protein architecture of the human kinetochore microtubule attachment site. Cell 137, 672–684 (2009).
Brinkley, B. R. & Stubblefield, E. The fine structure of the kinetochore of a mammalian cell in vitro. Chromosoma 19, 28–43 (1966).
McEwen, B. F., Ding, Y. & Heagle, A. B. Relevance of kinetochore size and microtubule-binding capacity for stable chromosome attachment during mitosis in PtK 1 cells. Chromosome Res. 6, 123–132 (1998).
Hoffman, D. B., Pearson, C. G., Yen, T. J., Howell, B. J. & Salmon, E. D. Microtubule-dependent changes in assembly of microtubule motor proteins and mitotic spindle checkpoint proteins at PtK 1 kinetochores. Mol. Biol. Cell 12, 1995–2009 (2001).
McEwen, B. F., Arena, J. T., Frank, J. & Rieder, C. L. Structure of the colcemid-treated PtK1 kinetochore outer plate as determined by high voltage electron microscopic tomography. J. Cell Biol. 120, 301–312 (1993).
Cimini, D. et al. Merotelic kinetochore orientation is a major mechanism of aneuploidy in mitotic mammalian tissue cells. J. Cell Biol. 153, 517–528 (2001).
Thrower, D. A., Jordan, M. A. & Wilson, L. Modulation of CENP-E organization at kinetochores by spindle microtubule attachment. Cell Motil. Cytoskeleton 35, 121–133 (1996).
Feng, J., Huang, H. & Yen, T. J. CENP-F is a novel microtubule-binding protein that is essential for kinetochore attachments and affects the duration of the mitotic checkpoint delay. Chromosoma 115, 320–329 (2006).
Cooke, C. A., Schaar, B., Yen, T. J. & Earnshaw, W. C. Localization of CENP-E in the fibrous corona and outer plate of mammalian kinetochores from prometaphase through anaphase. Chromosoma 106, 446–455 (1997).
Kim, Y., Heuser, J. E., Waterman-Storer, C. M. & Cleveland, D. W. CENP-E combines a slow, processive motor and a flexible coiled coil to produce an essential motile kinetochore tether. J. Cell Biol. 181, 411–419 (2008).
Magidson, V. et al. The spatial arrangement of chromosomes during prometaphase facilitates spindle assembly. Cell 146, 555–567 (2011).
Loncarek, J. et al. The centromere geometry essential for keeping mitosis error free is controlled by spindle forces. Nature 450, 745–749 (2007).
Wood, K. W. et al. Antitumor activity of an allosteric inhibitor of centromere-associated protein-E. Proc. Natl Acad. Sci. USA 107, 5839–5844 (2010).
Kapoor, T. M. et al. Chromosomes can congress to the metaphase plate before biorientation. Science 311, 388–391 (2006).
Cai, S., O’Connell, C. B., Khodjakov, A. & Walczak, C. E. Chromosome congression in the absence of kinetochore fibers. Nat. Cell Biol. 11, 832–838 (2009).
Shrestha, R. L. & Draviam, V. M. Lateral to end-on conversion of chromosome-microtubule attachment requires kinesins CENP-E and MCAK. Curr. Biol. 23, 1514–1526 (2013).
Collin, P., Nashchekina, O., Walker, R. & Pines, J. The spindle assembly checkpoint works like a rheostat rather than a toggle switch. Nat. Cell Biol. 15, 1378–1385 (2013).
Roos, U. P. Light and electron microscopy of rat kangaroo cells in mitosis. II. Kinetochore structure and function. Chromosoma 41, 195–220 (1973).
Goldstein, L. S. Kinetochore structure and its role in chromosome orientation during the first meiotic division in male D. melanogaster. Cell 25, 591–602 (1981).
Church, K. & Lin, H. P. Kinetochore microtubules and chromosome movement during prometaphase in Drosophila melanogaster spermatocytes studied in life and with the electron microscope. Chromosoma 92, 273–282 (1985).
Cimini, D., Moree, B., Canman, J. C. & Salmon, E. D. Merotelic kinetochore orientation occurs frequently during early mitosis in mammalian tissue cells and error correction is achieved by two different mechanisms. J. Cell Sci. 116, 4213–4225 (2003).
Wollman, R. et al. Efficient chromosome capture requires a bias in the ‘search-and-capture’ process during mitotic-spindle assembly. Curr. Biol. 15, 828–832 (2005).
Paul, R. et al. Computer simulations predict that chromosome movements and rotations accelerate mitotic spindle assembly without compromising accuracy. Proc. Natl Acad. Sci. USA 106, 15708–15713 (2009).
Holy, T. E. & Leibler, S. Dynamic instability of microtubules as an efficient way to search in space. Proc. Natl Acad. Sci. USA 91, 5682–5685 (1994).
Hill, T. L. Theoretical problems related to the attachment of microtubules to kinetochores. Proc. Natl Acad. Sci. USA 82, 4404–4408 (1985).
Jones, J. T., Myers, J. W., Ferrell, J. E. & Meyer, T. Probing the precision of the mitotic clock with a live-cell fluorescent biosensor. Nat. Biotechnol. 22, 306–312 (2004).
Rieder, C. L. & Alexander, S. P. Kinetochores are transported poleward along a single astral microtubule during chromosome attachment to the spindle in newt lung cells. J. Cell Biol. 110, 81–95 (1990).
Kitajima, T. S., Ohsugi, M. & Ellenberg, J. Complete kinetochore tracking reveals error-prone homologous chromosome biorientation in mammalian oocytes. Cell 146, 568–581 (2011).
DeLuca, J. G. et al. Hec1 and Nuf2 are core components of the kinetochore outer plate essential for organizing microtubule attachment sites. Mol. Biol. Cell 16, 519–531 (2005).
Lancaster, O. M. et al. Mitotic rounding alters cell geometry to ensure efficient bipolar spindle formation. Dev. Cell 25, 270–283 (2013).
O’Connell, C. B., Loncarek, J., Kalab, P. & Khodjakov, A. Relative contributions of chromatin and kinetochores to mitotic spindle assembly. J. Cell Biol. 187, 43–51 (2009).
Kalinina, I. et al. Pivoting of microtubules around the spindle pole accelerates kinetochore capture. Nat. Cell Biol. 15, 82–87 (2013).
Merdes, A. & De May, J. The mechanism of kinetochore-spindle attachment and polewards movement analyzed in PtK2 cells at the prophase-prometaphase transition. Eur. J. Cell Biol. 53, 313–325 (1990).
Nicholson, J. M. & Cimini, D. How mitotic errors contribute to karyotypic diversity in cancer. Adv. Cancer Res. 112, 43–75 (2011).
Silkworth, W. T. & Cimini, D. Transient defects of mitotic spindle geometry and chromosome segregation errors. Cell Div. 7, 19 (2012).
Mazumdar, M., Sundareshan, S. & Misteli, T. Human chromokinesin KIF4A functions in chromosome condensation and segregation. J. Cell Biol. 166, 613–620 (2004).
Wandke, C. et al. Human chromokinesins promote chromosome congression and spindle microtubule dynamics during mitosis. J. Cell Biol. 198, 847–863 (2012).
Bakhoum, S. F., Genovese, G. & Compton, D. A. Deviant kinetochore-microtubule dynamics underlie chromosomal instability. Curr. Biol. 19, 1937–1942 (2009).
Ganem, N. J., Godinho, S. A. & Pellman, D. A mechanism linking extra centrosomes to chromosomal instability. Nature 460, 278–282 (2009).
Silkworth, W. T., Nardi, I. K., Scholl, L. M. & Cimini, D. Multipolar spindle pole coalescence is a major source of kinetochore mis-attachment and chromosome mis-segregation in cancer cells. PLoS ONE 4, e6564 (2009).
Kleylein-Sohn, J. et al. Acentrosomal spindle organization renders cancer cells dependent on the kinesin HSET. J. Cell Sci. 125, 5391–402 (2012).
Sikirzhytski, V. et al. Direct kinetochore-spindle pole connections are not required for chromosome segregation. J. Cell Biol. 206, 231–243 (2014).
Le Berre, M., Aubertin, J. & Piel, M. Fine control of nuclear confinement identifies a threshold deformation leading to lamina rupture and induction of specific genes. Integr. Biol. 4, 1406–1414 (2012).
Kline, S. L., Cheeseman, I. M., Hori, T., Fukagawa, T. & Desai, A. The human Mis12 complex is required for kinetochore assembly and proper chromosome segregation. J. Cell Biol. 173, 9–17 (2006).
Rieder, C. L. & Cassels, G. Correlative light and electron microscopy of mitotic cells in monolayer cultures. Methods Cell Biol. 61, 297–315 (1999).
Acknowledgements
This work was supported by NIH grant GM059363 to A.K. and NSF grant DMS-1118206 to A.M. The Electron Microscopy was enabled by the use of the Wadsworth Center’s Electron Microscopy Core Facility. We thank J. Pines (University of Cambridge, UK) for his generous donation of Mad2–Venus cells and S. Li (Air Worldwide, USA) for assistance with the intensity quantifications.
Author information
Authors and Affiliations
Contributions
A.K. and B.F.M. designed the experiments, V.M., N.Y., C.B.O’C. and I.T. performed the experiments. J.G.A., B.F.M., A.K. and I.T. conducted correlative LM/EM. A.M. and R.P. developed the computational models. R.P. designed computer code and performed simulations. The manuscript was written by A.K., B.F.M. and A.M.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
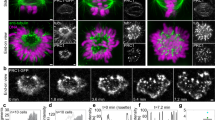
Supplementary Figure 2 Experimental approach used to characterize the ‘virgin’ kinetochore architecture at spindle assembly onset.
(a) Selected differential interference contrast (DIC) images (individual planes) illustrating a prophase cell immediately before the addition of 3-μM nocodazole (0 min) and at nuclear envelope breakdown (NEB, 6 min). The cell was fixed with 1% glutaraldehyde immediately after NEB. (b) A single optical plane near the middle of the cell shown after fixation in DIC and fluorescence. Notice that chromosomes (blue, Hoechst 33343) appear to be still aligned along the remnants of the nuclear envelope (arrows) indicating that the cell was fixed at the onset of mitosis. (c) Maximal intensity projection of the entire cell. (d) Higher magnification view of kinetochores from the boxed area in (c). The outer layer (red, CenpF) is enlarged and largely encircles the centromere. Inner kinetochores (green, CenpA-GFP) remain compact. Maximal intensity projections of a local sub-volume and surface-rendered reconstruction segmented at 25% of maximal intensity. (e,f) Treatment history of the cell shown in Fig. 3e, f. (e) Selected DIC and corresponding fluorescent images (CenpA-GFP and Centrin-GFP) depicting a different cell before the addition of 3-μM nocodazole (0 min), during late prophase (4 min), at (NEB, 10 min), and immediately after fixation (11 min). Asterisks indicate the location of the centrioles. The boxed area in panels D and E is shown in Fig. 2B at higher magnification. (f) Electron-microscopy image of the same cell. Remnants of the nuclear envelope are clearly visible (yellow arrows). The box denotes the centromere presented in Fig. 2b.
Supplementary Figure 3 Kinetochore outer layer compaction correlates with the formation of end-on microtubule attachment.
(a) In earlier prometaphase cells, kinetochores with an enlarged outer layer are present throughout the cell (insets 1, 2). However, a few kinetochores that display prominent end-on attachment to microtubule bundles are compact (insets 3, 4). (b) In later prometaphase, at least one kinetochore displays an enlarged outer layer on each monooriented chromosome and these enlarged kinetochores lack end-on microtubule attachments (insets 1, 2). In contrast, both sister kinetochores on bioriented chromosomes are compact (insets 3, 4). Whole-cell images are maximal-intensity projections that include all kinetochores in the cell. Individual kinetochores are shown as maximal-intensity projections of local sub-volumes.
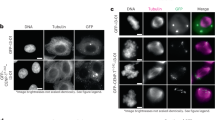
Supplementary Figure 4 Distribution and amounts of various kinetochore proteins in the absence of microtubules.
(a) Maximal intensity projections (include all kinetochores) depicting RPE1 cells after 15-min exposure to 3-μM nocodazole. Notice that the analyses were only on cells that had formed a metaphase plate before the addition of nocodazole as evident from the pattern of chromosome distribution and positions of centrosomes on the opposite sides of the plates. (b) Examples of individual kinetochores from the boxed areas in (a), shown at higher magnification. CenpF forms large crescents that can completely encircle the centromere. The distributions of Hec1 and Mis12 also appear to broaden albeit to lesser extents than CenpF. (c) Relative fluorescence intensities of kinetochores at various times after addition of nocodazole. The amount of the outer layer protein CenpF (red) remains at the level typical for untreated metaphase (compare with. Figure 1c, p > 0.3, two-tailed Student’s t-test for both blue versus blue and yellow versus yellow bars.) and then increases approximately threefold. The amount of Hec1 instantly increases approximately twofold over the levels typical for kinetochores during metaphase (compare with Fig. 1c, p < 0.0001, two-tailed Student’s test for both blue versus blue and yellow versus yellow bars.). The amount of Mis12 in nocodazole-treated cells is not significantly different from the metaphase level (compare with Fig. 1c, p > 0.09, two-tailed Student’s test for both blue versus blue and yellow versus yellow bars). Blue bars in (c) are mean kinetochore intensity calculated as mean of mean values for multiple kinetochores in individualcells (n values above the bars, Cs; cells) Error bars represent s.e.m. Yellow bars are mean values calculated for all kinetochores pooled from all cells in that class (n values above the bars, Ks; kinetochores). Error bars represent s.d.
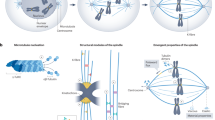
Supplementary Figure 5 Effects of kinetochore enlargement-compaction on the efficiency and fidelity of capture-driven spindle assembly.
(a–a′) Architecture of the virgin (unattached) centromere considered in the previous computational models of spindle assembly (a) versus the current model (a′). dKt, diameter of the discoid kinetochore in previous models; wKt and hKt, width and height of the expanded crescent-like kinetochore; Gap, segment of the centromere not covered by the kinetochore outer layer. (b) Diagram showing the changes in the centromere architecture considered in the minimalistic computational model. τcapt, time from the onset of spindle assembly to end-on attachment; τcomp, duration of the conversion from the expanded crescent to compact architecture of the kinetochore. (c) Sequence of events envisioned in the minimalistic model. Attachment triggers kinetochore compaction but does not affect orientation of the centromere. Green lines represent properly attached microtubules, red lines—potential erroneous attachments. (d,d′) Duration of spindle assembly and frequency of errors predicted for various final gap sizes, and various durations of kinetochore compaction. Notice that both efficiency and accuracy of spindle assembly remain nearly constant at τcomp > 60 s. (e) Frequency of errors and duration of spindle assembly predicted for centromeres with various final gap sizes for specific values of τexpand and τcomp.
Supplementary Figure 6 Rotation of the centromere due to lateral interactions between kinetochores and microtubules.
(a) Opposition of inward-directed forces generated at the kinetochore (dynein) and outward forces acting on chromosome arms (chromokinesins) rotate the centromere positioned on the surface of the spindle. (b) Centromeres with a small gap between sister kinetochores can rotate significantly while maintaining constant contact with microtubules. In contrast, rotation of centromeres with a large gap between sister kinetochores is sterically limited due to small kinetochores losing direct contact with microtubules. As the result, after rotation large sister kinetochores are primarily exposed to their proximal poles (green arrows) and shielded from the distal poles by the centromere. Due to the lesser angular improvement, smaller sister kinetochores remain exposed to both proximal and distal poles (red arrows).
Supplementary Figure 7 Conventional (not rotationally aligned) views of the cells presented in Fig. 8.
(a) Normal spindle assembly in an RPE1 cell (see Fig. 7a). (b) An untreated RPE1 cell with lagging chromosomes (Fig. 7b). (c) An RPE1 cell that assembled its spindle after nocodazole washout. Notice that drug washout is initiated soon after NEB when remnants of the nuclear envelope are still present in the cell (arrowheads). Each time point is shown in DIC (medial slices from 3D volumes) and GFP-fluorescence (maximal intensity projections). Asterisks denote mother centrioles (labeled with centrin-GFP). Arrows point at NEB remnants in DIC images and lagging chromosomes in fluorescent images. Time in minutes: seconds from NEB (a,b) or from completion of nocodazole washout (c).
Supplementary information
Supplementary Information
Supplementary Information (PDF 683 kb)
Supplementary Computer Code
Supplementary Information (TXT 96 kb)
Incorporation of individual chromosomes into the spindle. Related to Fig. 6.
Mad2 fluorescence (green) is overlaid on phase contrast (grey). The video starts with 3 centromeres on the lower left side of the spindle showing Mad2 fluorescence. The chromosome nearest the spindle equator has already initiated congression at the start of filming and rapidly moves to the equator where it loses Mad2 fluorescence. The other two centromeres show one or more rapid rotations (lasting no more than two consecutive frames at 30-s intervals) before initiating congression and loss of Mad2 fluorescence. Time in minutes: seconds from the start of the video. (MOV 1632 kb)
Axial and transverse views of spindle assembly in control and nocodazole treated cells. Related to Fig. 8.
A typical control cell is shown in the left panels, a control cell with lagging chromosomes in the middle panels, and a cell subjected to nocodazole treatment followed by washout of the drug in the right panels. Note that the typical control cell forms the clear middle zone with all centromeres confined to the periphery (frames 3–13). The fluorescent dots in the middle of the transverse views are the centrioles. Time in minutes : seconds from NEB (left and central panels) or from completion of nocodazole washout (right panel). (MOV 3653 kb)
A typical control cell as conventionally seen in the light microscope (not rotationally aligned).
The video shows the medial plane of DIC and the maximal-intensity projection of GFP-fluorescence for the cell shown in Fig. 8a and the left panels of Supplementary Video 2 . Note that clear zone formation in the central spindle is not evident in this view. Time in minutes: seconds from NEB. (MOV 5145 kb)
A control cell with lagging and lost chromosomes as conventionally seen in the light microscope (not rotationally aligned).
The video shows the medial plane of DIC and the maximal-intensity projection of GFP-fluorescence for the cell shown in Fig. 8b and the middle panels of Supplementary Video 2 . Note that the difference in clear zone formation between a typical control cell (Supplementary Video 3) and one leading to lost chromosomes (this video) is not detected. Hence, detection of the lack of clear zone formation requires that data sets be rotationally aligned. Time in minutes: seconds from NEB. (MOV 4934 kb)
A cell treated with nocodazole in prophase followed by drug washout after NEB as conventionally seen in the light microscope (not rotationally aligned).
The video shows the medial plane of DIC and the maximal-intensity projection of GFP-fluorescence for the cell shown in Fig. 8c and the right panels of Supplementary Video 2 . As in Supplementary Video 4, the lack of a clear zone is not detected unless data sets are rotationally aligned. Time in minutes: seconds from completion of nocodazole washout. (MOV 6000 kb)
Rights and permissions
About this article
Cite this article
Magidson, V., Paul, R., Yang, N. et al. Adaptive changes in the kinetochore architecture facilitate proper spindle assembly. Nat Cell Biol 17, 1134–1144 (2015). https://doi.org/10.1038/ncb3223
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3223
This article is cited by
-
CENP-E activation by Aurora A and B controls kinetochore fibrous corona disassembly
Nature Communications (2023)
-
Mechanisms underlying spindle assembly and robustness
Nature Reviews Molecular Cell Biology (2023)
-
E3-ubiquitin ligase, FBXW7 regulates mitotic progression by targeting BubR1 for ubiquitin-mediated degradation
Cellular and Molecular Life Sciences (2023)
-
Mechanical Torque Promotes Bipolarity of the Mitotic Spindle Through Multi-centrosomal Clustering
Bulletin of Mathematical Biology (2022)
-
Counteraction between Astrin-PP1 and Cyclin-B-CDK1 pathways protects chromosome-microtubule attachments independent of biorientation
Nature Communications (2021)