Abstract
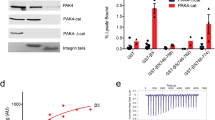
A-kinase anchoring proteins (AKAPs) control the localization and substrate specificity of cAMP-dependent protein kinase (PKA), tetramers of regulatory (PKA-R) and catalytic (PKA-C) subunits, by binding to PKA-R subunits1. Most mammalian AKAPs bind Type II PKA through PKA-RII (ref. 2), whereas dual specificity AKAPs bind both PKA-RI and PKA-RII (ref. 3). Inhibition of PKA–AKAP interactions modulates PKA signalling2. Localized PKA activation in pseudopodia of migrating cells4 phosphorylates α4 integrins to provide spatial cues governing cell motility5. Here, we report that the α4 cytoplasmic domain is a Type I PKA-specific AKAP that is distinct from canonical AKAPs in two ways: the α4 interaction requires the PKA holoenzyme, and is insensitive to amphipathic peptides that disrupt most PKA–AKAP interactions. We exploited type-specific PKA anchoring peptides to create genetically encoded baits that sequester specific PKA isoforms to the mitochondria and found that mislocalization of Type I, but not Type II, PKA disrupts α4 phosphorylation and markedly inhibits the velocity and directional persistence of cell migration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Carr, D. W. et al. Interaction of the regulatory subunit (RII) of cAMP-dependent protein kinase with RII-anchoring proteins occurs through an amphipathic helix binding motif. J. Biol. Chem. 14188–14192 (1991).
Wong, W. & Scott, J. D. AKAP signalling complexes: focal points in space and time. Nature Rev. Mol. Cell Biol. 5, 959–970 (2004).
Wang, L. et al. Cloning and mitochondrial localization of full-length D-AKAP2, a protein kinase A anchoring protein. Proc. Natl Acad. Sci. USA 98, 3220–3225 (2001).
Howe, A. K., Baldor, L. C. & Hogan, B. P. Spatial regulation of the cAMP-dependent protein kinase during chemotactic cell migration. Proc. Natl Acad. Sci. USA 102, 14320–14325 (2005).
Goldfinger, L. E., Han, J., Kiosses, W. B., Howe, A. K. & Ginsberg, M. H. Spatial restriction of α4 integrin phosphorylation regulates lamellipodial stability and α4β1-dependent cell migration. J. Cell Biol. 162, 731–741 (2003).
Liu, S. et al. Binding of paxillin to α4 integrins modifies integrin-dependent biological responses. Nature 402, 676–681 (1999).
Amieux, P. S. et al. Increased basal cAMP-dependent protein kinase activity inhibits the formation of mesoderm-derived structures in the developing mouse embryo. J. Biol. Chem. 277, 27294–27304 (2002).
Taylor, S. S., Buechler, J. A. & Yonemoto, W. cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu. Rev. Biochem. 59, 971–1005 (1990).
Burns-Hamuro, L. L. et al. Designing isoform-specific peptide disruptors of protein kinase A localization. Proc. Natl Acad. Sci. USA 100, 4072–4077 (2003).
Bear, J. E. et al. Negative regulation of fibroblast motility by Ena/VASP proteins. Cell 101, 717–728 (2000).
Mandeville, J. T., Ghosh, R. N. & Maxfield, F. R. Intracellular calcium levels correlate with speed and persistent forward motion in migrating neutrophils. Biophys. J. 68, 1207–1217 (1995).
Nishiya, N., Kiosses, W. B., Han, J. & Ginsberg, M. H. An α4 integrin–paxillin–Arf–GAP complex restricts Rac activation to the leading edge of migrating cells. Nature Cell Biol. 7, 343–352 (2005).
Cummings, D. E. et al. Genetically lean mice result from targeted disruption of the RIIβ subunit of protein kinase A. Nature 382, 622–626 (1996).
Viste, K., Kopperud, R. K., Christensen, A. E. & Doskeland, S. O. Substrate enhances the sensitivity of type I protein kinase A to cAMP. J. Biol. Chem. 280, 13279–13284 (2005).
Aandahl, E. M. et al. Inhibition of antigen-specific T cell proliferation and cytokine production by protein kinase A type I. J. Immunol. 169, 802–808 (2002).
Carrasco, Y. R. & Batista, F. D. B-cell activation by membrane-bound antigens is facilitated by the interaction of VLA-4 with VCAM-1. EMBO J. 25, 889–899 (2006).
Casey, M. et al. Mutations in the protein kinase A R1α regulatory subunit cause familial cardiac myxomas and Carney complex. J. Clin. Invest. 106, R31–R38 (2000).
Kirschner, L. S. et al. Mutations of the gene encoding the protein kinase A type I-α regulatory subunit in patients with the Carney complex. Nature Genet. 26, 89–92 (2000).
von Andrian, U. H. & Engelhardt, B. α4 integrins as therapeutic targets in autoimmune disease. N. Engl. J. Med. 348, 68–72 (2003).
Feral, C. C. et al. Blocking the α4 integrin–paxillin interaction selectively impairs mononuclear leukocyte recruitment to an inflammatory site. J. Clin. Invest 116, 715–723 (2006).
Arroyo, A. G., Yang, J. T., Rayburn, H. & Hynes, R. O. Differential requirements for α4 integrins during fetal and adult hematopoiesis. Cell 85, 997–1008 (1996).
Rose, D. M. et al. Paxillin binding to the alpha 4 integrin subunit stimulates LFA-1 (integrin αLα2)-dependent T cell migration by augmenting the activation of focal adhesion kinase/proline-rich tyrosine kinase-2. J. Immunol. 170, 5912–5918 (2003).
Arias-Salgado, E. G. et al. Src kinase activation by direct interaction with the integrin β cytoplasmic domain. Proc. Natl Acad. Sci. USA 100, 13298–13302 (2003).
Anand, G. S., Hughes, C. A., Jones, J. M., Taylor, S. S. & Komives, E. A. Amide H/2H exchange reveals communication between the cAMP and catalytic subunit-binding sites in the R(I) α subunit of protein kinase A. J. Mol. Biol. 323, 377–386 (2002).
Canaves, J. M., Leon, D. A. & Taylor, S. S. Consequences of cAMP-binding site mutations on the structural stability of the type I regulatory subunit of cAMP-dependent protein kinase. Biochemistry 39, 15022–15031 (2000).
Kim, C., Xuong, N. H. & Taylor, S. S. Crystal structure of a complex between the catalytic and regulatory (RIα) subunits of PKA. Science 307, 690–696 (2005).
Carlson, C. R., Ruppelt, A. & Tasken, K. A kinase anchoring protein (AKAP) interaction and dimerization of the RIα and RIβ regulatory subunits of protein kinase A in vivo by the yeast two hybrid system. J. Mol. Biol. 327, 609–618 (2003).
Iyer, G. H., Moore, M. J. & Taylor, S. S. Consequences of lysine 72 mutation on the phosphorylation and activation state of cAMP-dependent kinase. J. Biol. Chem. 280, 8800–8807 (2005).
Kammerer, S. et al. Amino acid variant in the kinase binding domain of dual-specific A kinase-anchoring protein 2: a disease susceptibility polymorphism. Proc. Natl Acad. Sci. USA 100, 4066–4071 (2003).
Acknowledgements
We gratefully acknowledge L. Goldfinger and N. Nishiya for helpful discussions, and M. Deal for technical assistance. This work was supported by grants from the National Institutes of Health (NIH) to M.H.G., the Leukemia and Lymphoma Society to C.J.L., and Arthritis Foundation to J.H.
Author information
Authors and Affiliations
Contributions
C.J.L. performed all experiments with contributions from J.H., N.Y. and P.S.A. C.J.L., J.H. and M.H.G. planned and interpreted experimental data. Y.M., S.S.T., P.S.A. and G.S.K. provided critical reagents. C.J.L., M.H.G., S.S.T and G.S.K. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary figures S1, S2, S3, S4, S5 and S6 (PDF 560 kb)
Supplementary Information
Supplementary Movie 1 (AVI 3019 kb)
Rights and permissions
About this article
Cite this article
Lim, C., Han, J., Yousefi, N. et al. α4 Integrins are Type I cAMP-dependent protein kinase-anchoring proteins. Nat Cell Biol 9, 415–421 (2007). https://doi.org/10.1038/ncb1561
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1561