Abstract
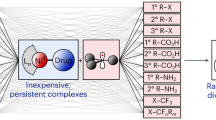
The olefin metathesis reaction of two unsaturated substrates is one of the most powerful carbon–carbon-bond-forming reactions in organic chemistry. Specifically, the catalytic olefin metathesis reaction has led to profound developments in the synthesis of molecules relevant to the petroleum, materials, agricultural and pharmaceutical industries1. These reactions are characterized by their use of discrete metal alkylidene catalysts that operate via a well-established mechanism2. While the corresponding carbonyl–olefin metathesis reaction can also be used to construct carbon–carbon bonds, currently available methods are scarce and severely hampered by either harsh reaction conditions or the required use of stoichiometric transition metals as reagents. To date, no general protocol for catalytic carbonyl–olefin metathesis has been reported. Here we demonstrate a catalytic carbonyl–olefin ring-closing metathesis reaction that uses iron, an Earth-abundant and environmentally benign transition metal, as a catalyst. This transformation accommodates a variety of substrates and is distinguished by its operational simplicity, mild reaction conditions, high functional-group tolerance, and amenability to gram-scale synthesis. We anticipate that these characteristics, coupled with the efficiency of this reaction, will allow for further advances in areas that have historically been enhanced by olefin metathesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hoveyda, A. H. & Zhugralin, A. R. The remarkable metal-catalysed olefin metathesis reaction. Nature 450, 243–251 (2007)
Grubbs, R. H. & Wenzel, A. G. Handbook of Metathesis 2nd edn, Vols 1–3 (Wiley, 2015)
Katz, T. J. & McGinnis, J. The mechanism of the olefin metathesis reaction. J. Am. Chem. Soc. 97, 1592–1594 (1975)
Grubbs, R. H., Carr, D. D., Hoppin, C. & Burk, P. L. Consideration of the mechanism of the metal catalyzed olefin metathesis reaction. J. Am. Chem. Soc. 98, 3478–3483 (1976)
Chauvin, Y. Olefin metathesis: the early days. Angew. Chem. Int. Ed. 45, 3740–3747 (2006)
Fürstner, A. Olefin metathesis and beyond. Angew. Chem. Int. Ed. 39, 3012–3043 (2000)
Maryanoff, B. E. & Reitz, A. B. The Wittig olefination reaction and modifications involving phosphoryl-stabilized carbanions. Stereochemistry, mechanism, and selected synthetic aspects. Chem. Rev. 89, 863–927 (1989)
Petasis, N. A. & Bzowej, E. I. Titanium-mediated carbonyl olefinations. 1. Methylenations of carbonyl compounds with dimethyltitanocene. J. Am. Chem. Soc. 112, 6392–6394 (1990)
Takeda, T. & Tsubouchi, A. in Modern Carbonyl Olefination (ed. Takeda, T. ) 151–199 (Wiley, 2003)
Jones, G. II, Acquadro, M. A. & Carmody, M. A. Long-chain enals via carbonyl–olefin metathesis. An application in pheromone synthesis. J. Chem. Soc. Chem. Commun. 6, 206–207 (1975)
Pérez-Ruiz, R., Miranda, M. A., Alle, R., Meerholz, K. & Griesbeck, A. G. An efficient carbonyl-alkene metathesis of bicyclic oxetanes: photoinduced electron transfer reduction of the Paternò–Büchi adducts from 2,3-dihydrofuran and aromatic aldehydes. Photochem. Photobiol. Sci. 5, 51–55 (2006)
Valiulin, R. A. & Kutateladze, A. G. Harvesting the strain installed by a Paternò−Büchi step in a synthetically useful way: high-yielding photoprotolytic oxametathesis in polycyclic systems. Org. Lett. 11, 3886–3889 (2009)
Fu, G. C. & Grubbs, R. H. Synthesis of cycloalkenes via alkylidene-mediated olefin metathesis and carbonyl olefination. J. Am. Chem. Soc. 115, 3800–3801 (1993)
Stille, J. R., Santarsiero, B. D. & Grubbs, R. H. Rearrangement of bicyclo[2.2.1]heptane ring systems by titanocene alkylidene complexes to bicyclo[3.2.0]heptane enol ethers. Total synthesis of (±)-Δ9(12)-capnellene. J. Org. Chem. 55, 843–862 (1990)
Iyer, K. & Rainier, J. D. Olefinic ester and diene ring-closing metathesis using a reduced titanium alkylidene. J. Am. Chem. Soc. 129, 12604–12605 (2007)
Soicke, A., Slavov, N., Neudörfl, J.-M. & Schmalz, H.-G. Metal-free intramolecular carbonyl–olefin metathesis of ortho-prenylaryl ketones. Synlett 17, 2487–2490 (2011)
van Schaik, H.-P., Vijn, R.-J. & Bickelhaupt, F. Acid-catalyzed olefination of benzaldehyde. Angew. Chem. Int. Edn Engl. 33, 1611–1612 (1994)
Bah, J., Franzén, J. & Naidu, V. R. Direct organocatalytic oxo-metathesis, a trans-selective carbocation-catalyzed olefination of aldehydes. Eur. J. Org. Chem. 2015, 1834–1839 (2015)
Jossifov, C., Kalinova, R. & Demonceau, A. Carbonyl olefin metathesis. Chim. Oggi 26, 85–87 (2008)
Griffith, A. K., Vanos, C. M. & Lambert, T. H. Organocatalytic carbonyl-olefin metathesis. J. Am. Chem. Soc. 134, 18581–18584 (2012)
Hong, B., Li, H., Wu, J., Zhang, J. & Lei, X. Total syntheses of (−)-huperzine Q and (+)-lycopladines B and C. Angew. Chem. Int. Ed. 54, 1011–1015 (2015)
Heller, S. T., Kiho, T., Narayan, A. R. H. & Sarpong, R. Protic-solvent-mediated cycloisomerization of quinoline and isoquinoline propargylic alcohols: syntheses of (±)-3-demethoxyerythratidinone and (±)-cocculidine. Angew. Chem. Int. Ed. 52, 11129–11133 (2013)
Clarke, M. L. & France, M. B. The carbonyl ene reaction. Tetrahedron 64, 9003–9031 (2008)
Miles, R. B., Davis, C. E. & Coates, R. M. Syn- and anti-selective Prins cyclizations of δ,ε-unsaturated ketones to 1,3-halohydrins with Lewis acids. J. Org. Chem. 71, 1493–1501 (2006)
Jackson, A. C., Goldman, B. E. & Snider, B. B. Intramolecular and intermolecular Lewis acid catalyzed ene reactions using ketones as enophiles. J. Org. Chem. 49, 3988–3994 (1984)
Reetz, M. T. Lewis acid induced α-alkylation of carbonyl compounds. Angew. Chem. Int. Edn Engl. 21, 96–108 (1982)
Demole, E., Enggist, P. & Borer, M. C. Applications synthétiques de la cyclisation d’alcools tertiaires γ-éthyléniques en α-bromotétrahydrofurannes sous l’action du N-bromosuccinimide. II. Cyclisation du (±)-nérolidol en diméthyl-2,5-(méthyl-4-pentène-3-yl)-2-cycloheptène-4-one, tétraméthyl-3, 3, 7, 10-oxa-2-tricyclo[5.5.0.01,4]-dodécène-9, β-acoratriène, cédradiène-2,8, épi-2-α-cédrène et α-cédrène. Helv. Chim. Acta 54, 1845–1864 (1971)
Carless, H. A. J. & Trivedi, H. S. New ring expansion reaction of 2-t-butyloxetans. J. Chem. Soc. Chem. Commun. 382–383 (1979)
Zimmerman, P. M. Automated discovery of chemically reasonable elementary reaction steps. J. Comput. Chem. 34, 1385–1392 (2013)
Zimmerman, P. M. Reliable transition state searches integrated with the growing string method. J. Chem. Theory Comput. 9, 3043–3050 (2013)
Acknowledgements
This work was supported by the Petroleum Research Fund (PRF#54688-DNI1) and start-up funds provided by the University of Michigan. J.R.L. thanks the National Science Foundation for a predoctoral fellowship. P.M.Z. thanks the Office of Naval Research for support under grant N00014-14-1-0551 and the Petroleum Research Fund (PRF#54267-DNI6). We are grateful to A. Speelman, A. McQuarters and N. Lehnert at the University of Michigan for helpful discussions.
Author information
Authors and Affiliations
Contributions
J.R.L., J.B.G. and C.S.S. devised the experiments, prepared the starting materials and the products. P.M.Z. conducted the theoretical investigations. J.R.L., J.B.G., P.M.Z. and C.S.S. prepared this manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Tables 1-8 and 6 Supplementary Figures. Also included are Supplementary Methods and Materials, Supplementary Results, X-Ray crystallographic data and NMR Spectral data, which contains 1H and 13C NMR data for all new compounds. (PDF 34111 kb)
Rights and permissions
About this article
Cite this article
Ludwig, J., Zimmerman, P., Gianino, J. et al. Iron(III)-catalysed carbonyl–olefin metathesis. Nature 533, 374–379 (2016). https://doi.org/10.1038/nature17432
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature17432
This article is cited by
-
Carbonyl cross-metathesis via deoxygenative gem-di-metal catalysis
Nature Chemistry (2024)
-
Ring-closing C–O/C–O metathesis of ethers with primary aliphatic alcohols
Nature Communications (2023)
-
Azoarene activation for Schmidt-type reaction and mechanistic insights
Nature Communications (2022)
-
Autonomous Reaction Network Exploration in Homogeneous and Heterogeneous Catalysis
Topics in Catalysis (2022)
-
Simultaneously improving reaction coverage and computational cost in automated reaction prediction tasks
Nature Computational Science (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.