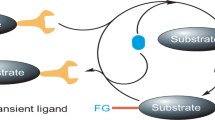
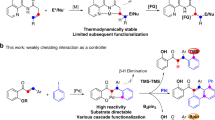
Abstract
Achieving site selectivity in C–H functionalization reactions is a significant challenge, especially when the target C–H bond is distant from existing functional groups1,2,3,4,5. Coordination of a functional group to a metal is often a key driving force and control element in many important reactions including asymmetric hydrogenation6, epoxidation7,8 and lithiation9. Exploitation of this effect has led to the development of a broad range of directed C–H activation reactions10,11,12,13,14. However, these C–H activation methods are limited to proximal C–H bonds, which are spatially and geometrically accessible from the directing functional group. The development of meta-selective C–H functionalizations remains a significant challenge1,2,3,4,5,15,16,17. We recently developed a U-shaped template that can be used to overcome this constraint and have shown that it can be used to selectively activate remote meta-C–H bonds1,2. Although this approach has proved to be applicable to various substrates and catalytic transformations3,4,5, the need for a covalently attached, complex template is a substantial drawback for synthetic applications. Here we report an alternative approach employing norbornene as a transient mediator to achieve meta-selective C–H activation with a simple and common ortho-directing group. The use of a newly developed pyridine-based ligand is crucial for relaying the palladium catalyst to the meta position by norbornene after initial ortho-C–H activation. This catalytic reaction demonstrates the feasibility of switching ortho-selectivity to meta-selectivity in C–H activation of the same substrate by catalyst control.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Leow, D., Li, G., Mei, T.-S. & Yu, J.-Q. Activation of remote meta-C–H bonds assisted by an end-on template. Nature 486, 518–522 (2012)
Tang, R.-Y., Li, G. & Yu, J.-Q. Conformation-induced remote meta-C–H activation of amines. Nature 507, 215–220 (2014)
Lee, S., Lee, H. & Tan, K. L. Meta-selective C–H functionalization using a nitrile-based directing group and cleavable Si-tether. J. Am. Chem. Soc. 135, 18778–18781 (2013)
Wan, L., Dastbaravardeh, N., Li, G. & Yu, J.-Q. Cross-coupling of remote meta-C–H bonds directed by a U-shaped template. J. Am. Chem. Soc. 135, 18056–18059 (2013)
Yang, G. et al. Pd(II)-catalyzed meta-C–H olefination, arylation, and acetoxylation of indolines using a U-shaped template. J. Am. Chem. Soc. 136, 10807–10813 (2014)
Brown, J. M. Selectivity and mechanism in catalytic asymmetric synthesis. Chem. Soc. Rev. 22, 25–41 (1993)
Johnson, R. A. & Sharpless, K. B. in Catalytic Asymmetric Synthesis 2nd edn (ed. Ojima, I.) 231–280 (Wiley, 2005)
Li, Z. & Yamamoto, H. Hydroxamic acids in asymmetric synthesis. Acc. Chem. Res. 46, 506–518 (2013)
Hartung, C. G. & Snieckus, V. in Modern Arene Chemistry (ed. Astruc, D.) 330–367 (Wiley-VCH, 2004)
Lyons, T. W. & Sanford, M. S. Palladium-catalyzed ligand-directed C–H functionalization reactions. Chem. Rev. 110, 1147–1169 (2010)
Daugulis, O., Do, H.-Q. & Shabashov, D. Palladium- and copper-catalyzed arylation of carbon–hydrogen bonds. Acc. Chem. Res. 42, 1074–1086 (2009)
Engle, K. M., Mei, T.-S., Wasa, M. & Yu, J.-Q. Weak coordination as a powerful means for developing broadly useful C–H functionalization reactions. Acc. Chem. Res. 45, 788–802 (2012)
Colby, D. A., Bergman, R. G. & Ellman, J. A. Rhodium-catalyzed C–C bond formation via heteroatom-directed C–H bond activation. Chem. Rev. 110, 624–655 (2010)
Wencel-Delord, J., Dröge, T., Liu, F. & Glorius, F. Towards mild metal-catalyzed C–H bond activation. Chem. Soc. Rev. 40, 4740–4761 (2011)
Saidi, O. et al. Ruthenium-catalyzed meta-sulfonation of 2-phenylpyridines. J. Am. Chem. Soc. 133, 19298–19301 (2011)
Duong, H. A., Gilligan, R. E., Cooke, M. L., Phipps, R. J. & Gaunt, M. J. Copper(II)-catalyzed meta-selective direct arylation of α-aryl carbonyl compounds. Angew. Chem. Int. Ed. 50, 463–466 (2011)
Hofmann, N. & Ackermann, L. meta-Selective C–H bond alkylation with secondary alkyl halides. J. Am. Chem. Soc. 135, 5877–5884 (2013)
Catellani, M., Frignani, F. & Rangoni, A. A complex catalytic cycle leading to a regioselective synthesis of o,o′-disubstituted vinylarenes. Angew. Chem. Int. Edn Engl. 36, 119–122 (1997)
Faccini, F., Motti, E. & Catellani, M. A new reaction sequence involving palladium-catalyzed unsymmetrical aryl coupling. J. Am. Chem. Soc. 126, 78–79 (2004)
Martins, A., Mariampillai, B. & Lautens, M. Synthesis in the key of Catellani: norbornene-mediated ortho C–H functionalization. Top. Curr. Chem. 292, 1–33 (2010)
Cárdenas, D. J., Martín-Matute, B. & Echavarren, A. M. Aryl transfer between Pd(II) centers or Pd(IV) intermediates in Pd-catalyzed domino reactions. J. Am. Chem. Soc. 128, 5033–5040 (2006)
Dong, Z. & Dong, G. Ortho vs ipso: site-selective Pd and norbornene-catalyzed arene C–H amination using aryl halides. J. Am. Chem. Soc. 135, 18350–18353 (2013)
Jiao, L. & Bach, T. Palladium-catalyzed direct 2-alkylation of indoles by norbornene-mediated regioselective cascade C–H activation. J. Am. Chem. Soc. 133, 12990–12993 (2011)
Jiao, L., Herdtweck, E. & Bach, T. Pd(II)-catalyzed regioselective 2-alkylation of indoles via a norbornene-mediated C–H activation: mechanism and applications. J. Am. Chem. Soc. 134, 14563–14572 (2012)
Catellani, M. & Ferioli, L. An improved synthesis of 1,4-cis,exo-hexa- or tetrahydromethano- or -ethanobiphenylene derivatives catalyzed by palladium complexes. Synthesis 769–772 (1996)
Wang, X.-C. et al. Pd(II)-catalyzed C−H iodination using molecular I2 as the sole oxidant. J. Am. Chem. Soc. 135, 10326–10329 (2013)
Zhu, R.-Y., He, J., Wang, X.-C. & Yu, J.-Q. Ligand-promoted alkylation of C(sp3)–H and C(sp2)–H bonds. J. Am. Chem. Soc. 136, 13194–13197 (2014)
He, J. et al. Ligand-controlled C(sp3)–H arylation and olefination in synthesis of unnatural chiral α–amino acids. Science 343, 1216–1220 (2014)
Li, S., Chen, G., Feng, C.-G., Gong, W. & Yu, J.-Q. Ligand-enabled γ-C–H olefination and carbonylation: construction of β-quaternary carbon centers. J. Am. Chem. Soc. 136, 5267–5270 (2014)
Martins, A., Candito, D. A. & Lautens, M. Palladium-catalyzed reductive ortho-arylation: evidence for the decomposition of 1,2-dimethoxyethane and subsequent arylpalladium(II) reduction. Org. Lett. 12, 5186–5188 (2010)
Acknowledgements
We thank J. Spangler and A. Homs for constructive suggestions. We thank the Scripps Research Institute and the National Institutes of Health (NIGMS, 1R01 GM102265) for financial support. L.-Z.F. is a visiting scholar from School of Pharmacy, Xinxiang Medical University and is sponsored by the China Scholarship Council.
Author information
Authors and Affiliations
Contributions
X.-C.W. and R.-Y.Z. developed the reactions. W.G. and S.L. synthesized the ligands. L.-Z.F. examined the substrate scope. K.M.E. performed preliminary studies. J.-Q.Y. conceived this concept and prepared the manuscript with feedback from X.-C.W.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data and Supplementary References, see contents page for details. (PDF 10759 kb)
Rights and permissions
About this article
Cite this article
Wang, XC., Gong, W., Fang, LZ. et al. Ligand-enabled meta-C–H activation using a transient mediator. Nature 519, 334–338 (2015). https://doi.org/10.1038/nature14214
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14214
This article is cited by
-
Unlocking regioselective meta-alkylation with epoxides and oxetanes via dynamic kinetic catalyst control
Nature Communications (2024)
-
N-heterocyclic carbene- and organic photoredox-catalysed meta-selective acylation of electron-rich arenes
Nature Synthesis (2023)
-
Efficient electrocatalytic valorization of chlorinated organic water pollutant to ethylene
Nature Nanotechnology (2023)
-
Synthesis of planar chiral ferrocenes via enantioselective remote C–H activation
Nature Chemistry (2023)
-
Traditional and sustainable approaches for the construction of C–C bonds by harnessing C–H arylation
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.