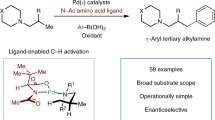
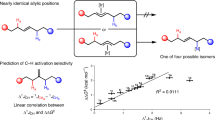
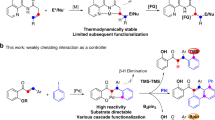
Abstract
The functionalization of C–H bonds in organic molecules is one of the most direct approaches for chemical synthesis. Recent advances in catalysis have allowed native chemical groups such as carboxylic acids, ketones and amines to control and direct C(sp3)–H activation1,2,3,4. However, alcohols, among the most common functionalities in organic chemistry5, have remained intractable because of their low affinity for late transition-metal catalysts6,7. Here we describe ligands that enable alcohol-directed arylation of δ-C(sp3)–H bonds. We use charge balance and a secondary-coordination-sphere hydrogen-bonding interaction—evidenced by structure–activity relationship studies, computational modelling and crystallographic data—to stabilize L-type hydroxyl coordination to palladium, thereby facilitating the assembly of the key C–H cleavage transition state. In contrast to previous studies in C–H activation, in which secondary interactions were used to control selectivity in the context of established reactivity8,9,10,11,12,13, this report demonstrates the feasibility of using secondary interactions to enable challenging, previously unknown reactivity by enhancing substrate–catalyst affinity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Crystallographic data for compounds 4a, 4r, 4am, S21 and S22 are available in the Supplementary Information files and from the Cambridge Crystallographic Data Center under reference numbers CCDC 2115513, CCDC 2263719, CCDC 2263720, CCDC 2265739 and CCDC 2265740, respectively. The structures of Pd complexes C1–C3 (see Supplementary Information) are also provided under reference numbers CCDC 2236162, CCDC 2236161 and CCDC 2236160. All other data supporting the findings of this study are available in the Article and its Supplementary Information files.
References
Chen, Z. et al. Transition metal-catalyzed C–H bond functionalizations by the use of diverse directing groups. Org. Chem. Front. 2, 1107–1295 (2015).
Sambiagio, C. et al. A comprehensive overview of directing groups applied in metal-catalysed C–H functionalisation chemistry. Chem. Soc. Rev. 47, 6603–6743 (2018).
Uttry, A. & van Gemmeren, M. Direct C(sp3)–H activation of carboxylic acids. Synthesis 52, 479–488 (2020).
Higham, J. I. & Bull, J. A. Transient imine directing groups for the C–H functionalisation of aldehydes, ketones and amines: an update 2018–2020. Org. Biomol. Chem. 18, 7291–7315 (2020).
Ertl, P. & Schuhmann, T. A systematic cheminformatics analysis of functional groups occurring in natural products. J. Nat. Prod. 82, 1258–1263 (2019).
Mo, F., Tabor, J. R. & Dong, G. Alcohols or masked alcohols as directing groups for C–H bond functionalization. Chem. Lett. 43, 264–271 (2014).
Vicente, J. & Arcas, A. Aqua palladium complexes: synthesis, properties and applications. Coord. Chem. Rev. 249, 1135–1154 (2005).
Kuninobu, Y., Ida, H., Nishi, M. & Kanai, M. A meta-selective C–H borylation directed by a secondary interaction between ligand and substrate. Nat. Chem. 7, 712–717 (2015).
Hoque, M. E., Bisht, R., Haldar, C. & Chattopadhyay, B. Noncovalent interactions in Ir-catalyzed C–H activation: L-shaped ligand for para-selective borylation of aromatic esters. J. Am. Chem. Soc. 139, 7745–7748 (2017).
Genov, G. R., Douthwaite, J. L., Lahdenperä, A. S. K., Gibson, D. C. & Phipps, R. J. Enantioselective remote C–H activation directed by a chiral cation. Science 367, 1246–1251 (2020).
Li, G., Yan, Y., Zhang, P., Xu, X. & Jin, Z. Palladium-catalyzed meta-selective C–H functionalization by noncovalent H-bonding interaction. ACS Catal. 11, 10460–10466 (2021).
Goswami, N. et al. Distal meta-alkenylation of formal amines enabled by catalytic use of hydrogen-bonding anionic ligands. Chem 9, 989–1003 (2023).
Mondal, A., Díaz-Ruiz, M., Deufel, F., Maseras, F. & van Gemmeren, M. Charge-controlled Pd catalysis enables the meta-C–H activation and olefination of arenes. Chem 9, 1004–1016 (2023).
Abrams, D. J., Provencher, P. A. & Sorensen, E. J. Recent applications of C–H functionalization in complex natural product synthesis. Chem. Soc. Rev. 47, 8925–8967 (2018).
Lam, N. Y. S., Wu, K. & Yu, J.-Q. Advancing the logic of chemical synthesis: C–H activation as strategic and tactical disconnections for C–C bond construction. Angew. Chem. Int. Ed. 60, 15767–15790 (2021).
Shao, Q., Wu, K., Zhuang, Z., Qian, S. & Yu, J.-Q. From Pd(OAc)2 to chiral catalysts: The discovery and development of bifunctional mono-N-protected amino acid ligands for diverse C–H functionalization reactions. Acc. Chem. Res. 53, 833–851 (2020).
Lucas, E. L. et al. Palladium-catalyzed enantioselective β-C(sp3)–H activation reactions of aliphatic acids: A retrosynthetic surrogate for enolate alkylation and conjugate addition. Acc. Chem. Res. 55, 537–550 (2022).
Park, H., Chekshin, N., Shen, P.-X. & Yu, J.-Q. Ligand-enabled, palladium-catalyzed β-C(sp3)–H arylation of Weinreb amides. ACS Catal. 8, 9292–9297 (2018).
Hoveyda, A. H., Evans, D. A. & Fu, G. C. Substrate-directable chemical reactions. Chem. Rev. 93, 1307–1370 (1993).
Terao, Y., Wakui, H., Satoh, T., Miura, M. & Nomura, M. Palladium-catalyzed arylative carbon–carbon bond cleavage of α,α-disubstituted arylmethanols. J. Am. Chem. Soc. 123, 10407–10408 (2001).
Terao, Y. et al. Palladium-catalyzed arylation of α,α-disubstituted arylmethanols via cleavage of a C–C or a C–H bond to give biaryls. J. Org. Chem. 68, 5236–5243 (2003).
Lu, Y., Wang, D.-H., Engle, K. M. & Yu, J.-Q. Pd(II)-catalyzed hydroxyl-directed C–H olefination enabled by monoprotected amino acid ligands. J. Am. Chem. Soc. 132, 5916–5921 (2010).
Wang, X., Lu, Y., Dai, H.-X. & Yu, J.-Q. Pd(II)-catalyzed hydroxyl-directed C–H activation/C–O cyclization: Expedient construction of dihydrobenzofurans. J. Am. Chem. Soc. 132, 12203–12205 (2010).
Lu, Y., Leow, D., Wang, X., Engle, K. M. & Yu, J.-Q. Hydroxyl-directed C–H carbonylation enabled by mono-N-protected amino acid ligands: an expedient route to 1-isochromanones. Chem. Sci. 2, 967–971 (2011).
Wen, Z.-K., Xu, Y.-H. & Loh, T.-P. Palladium-catalyzed cross-coupling of unactivated alkenes with acrylates: Application to the synthesis of the C13–C21 fragment of Palmerolide A. Chem. Eur. J. 42, 13284–13287 (2012).
Kandukuri, S. R., Jiao, L.-Y., Machotta, A. B. & Oestreich, M. Diastereotopic group selection in hydroxy-directed intramolecular C–H alkenylation of indole under oxidative palladium(II) catalysis. Adv. Synth. Catal. 356, 1597–1609 (2014).
Liang, Q.-J. et al. Chelation versus non-chelation control in the stereoselective alkenyl sp2 C–H bond functionalization reaction. Angew. Chem. Int. Ed. 56, 5091–5095 (2017).
Li, L., Liu, Q., Chen, J. & Huang, Y. Alcohol-directed ortho-C–H alkenylation. Synlett 30, 1366–1370 (2019).
Meng, K. et al. Geminal group-directed olefinic C–H functionalization via four- to eight-membered exo-metallocycles. Nat. Commun. 10, 5109 (2019).
Ghiringhelli, F., Uttry, A., Ghosh, K. K. & van Gemmeren, M. Direct β- and γ-C(sp3)–H alkynylation of free carboxylic acids. Angew. Chem. Int. Ed. 59, 23127–23131 (2020).
Bryndza, H. E. & Tam, W. Monomeric metal hydroxides, alkoxides, and amides of the late transition metals: Synthesis, reactions, and thermochemistry. Chem. Rev. 88, 1163–1188 (1988).
Fernández-Rivas, C. et al. Synthesis and structure of new oxapalladacycles with a Pd–O bond. Organometallics 20, 2998–3006 (2001).
Sigman, M. S. & Schultz, M. J. The renaissance of palladium(II)-catalyzed oxidation chemistry. Org. Biomol. Chem. 2, 2551–2554 (2004).
Xia, G. et al. Reversing conventional site-selectivity in C(sp3)–H bond activation. Nat. Chem. 11, 571–577 (2019).
Tanka, K., Ewing, W. R. & Yu, J.-Q. Hemilabile benzyl ether enables γ-C(sp3)–H carbonylation and olefination of alcohols. J. Am. Chem. Soc. 2019, 15494–15497 (2019).
Xia, G. et al. Ligand-enabled β-methylene C(sp3)–H arylation of masked aliphatic alcohols. Angew. Chem., Int. Ed. 59, 7783–7787 (2020).
Salamanca, V., Toledo, A. & Albéniz, A. C. [2,2’-Bipyridin]-6(1H)-one, a truly cooperating ligand in the palladium-mediated C–H activation step: experimental evidence in the direct C-3 arylation of pyridine. J. Am. Chem. Soc. 140, 17851–17856 (2018).
Li, Z. et al. A tautomeric ligand enables directed C–H hydroxylation with molecular oxygen. Science 372, 1452–1457 (2021).
Li, Z., Park, H. S., Qiao, J. X., Yeung, K.-S. & Yu, J.-Q. Ligand-enabled C–H hydroxylation with aqueous H2O2 at room temperature. J. Am. Chem. Soc. 144, 18109–18116 (2022).
Saint-Denis, T. G. et al. Mechanistic study of enantioselective Pd-catalyzed C(sp3)–H activation of thioethers involving two distinct stereomodels. ACS Catal. 11, 9738–9753 (2021).
Drover, M. W. A guide to secondary coordination sphere editing. Chem. Soc. Rev. 51, 1861–1880 (2022).
Wimmer, F. L., Wimmer, S., Afcharian, A., Castan, P. & Fabre, P. L. Acid dissociation and dimerization constants of some cis-diaqua complexes of palladium(II) with chelating N, N’ and N, C’ ligands. J. Chem. Res. Synop. 194–195 (1999).
Alsters, P. L., Boersma, J., Smeets, W. J. J., Spek, A. L. & van Koten, G. Arylpalladium compounds containing an alcohol functionality: Hindered rotation around the Pd–C bond and reactivity toward styrene and carbon monoxide. Comments on carbon–oxygen bond shortening in late-transition-metal alkoxides. Organometallics 12, 1639–1647 (1993).
Jie, S., Ai, P., Zhou, Q. & Li, B.-G. Nickel and cationic palladium complexes bearing (imino)pyridyl alcohol ligands: Synthesis, characterization and vinyl polymerization of norbornene. J. Organomet. Chem. 696, 1465–1473 (2011).
Reek, J. N. H. et al. Transition metal catalysis controlled by hydrogen bonding in the second coordination sphere. Chem. Rev. 122, 12308–12369 (2022).
Farizyan, M., Mondal, A., Mal, S., Deufel, F. & van Gemmeren, M. Palladium-catalyzed nondirected late-stage C–H deuteration of arenes. J. Am. Chem. Soc. 143, 16370–16373 (2021).
van der Kolk, M. R., Janssen, M. A. C. H., Rutjes, F. P. J. T. & Blanco-Ania, D. Cyclobutanes in small-molecule drug candidates. ChemMedChem 17, e202200020 (2022).
Frank, N. et al. Synthesis of meta-substituted arene bioisosteres from [3.1.1]propellane. Nature 611, 721–726 (2022).
Wang, Z. et al. Ligand-controlled divergent dehydrogenative reactions of carboxylic acids via C–H activation. Science 374, 1281–1285 (2021).
Cockroft, S. L. & Hunter, C. A. Chemical double-mutant cycles: dissecting non-covalent interactions. Chem. Soc. Rev. 36, 172–188 (2007).
Brazzolotto, D., Bogart, J. A., Ross, D. L., Ziller, J. W. & Borovik, A. S. Stabilizing a NiII-aqua complex via intramolecular hydrogen bonds: synthesis, structure, and redox properties. Inorg. Chim. Acta 495, 118960 (2019).
Knowles, R. R. & Jacobsen, E. N. Attractive noncovalent interactions in asymmetric catalysis: Links between enzymes and small molecule catalysts. Proc. Natl Acad. Sci. USA 107, 20678–20685 (2010).
Davis, H. J. & Phipps, R. J. Harnessing non-covalent interactions to exert control over regioselectivity and site-selectivity in catalytic reactions. Chem. Sci. 8, 864–877 (2017).
Neel, A. J., Hilton, M. J., Sigman, M. S. & Toste, F. D. Exploiting non-covalent π interactions for catalyst design. Nature 543, 637–646 (2017).
Hwang, J., Mercado, B. O. & Miller, S. J. Chirality-matched catalyst-controlled macrocyclization reactions. Proc. Natl Acad. Sci. USA 118, e2113122118 (2021).
Acknowledgements
We thank the Scripps Research Institute, the NIH (National Institute of General Medical Sciences grants R01 GM084019 and F32 GM143921) and the Jennifer and Dallas Luttrell Endowed Fellowship in the Skaggs Graduate School of Chemical and Biological Sciences for their financial support. The content is solely our responsibility and does not necessarily represent the official views of the National Institutes of Health. We thank K. Wu for assistance with automated conformational searches and for numerous discussions throughout the course of this project; M. Gembicky, J. Bailey, E. Samolova and the UCSD Crystallography Facility for X-ray crystallographic analysis; L. Pasternack, D.-H. Huang and G. Kroon of the Nuclear Magnetic Resonance Facility of the Scripps Researcher Services for their assistance with NMR analysis; B. Webb and E. Billings of the Scripps Center for Metabolomics and Mass Spectrometry and J. Chen, B. Sanchez and Q. N. Wong of the Scripps Automated Synthesis Facility for assistance with mass spectrometry. We acknowledge the group of M. van Gemmeren for taking the time to independently verify the reproducibility of the results obtained in this study.
Author information
Authors and Affiliations
Contributions
D.A.S. and J.-Q.Y. conceived of the project. D.A.S. developed the arylation of benzylic C–H bonds. D.A.S. and C.-Y.C. developed the arylation of cyclobutyl substrates and C.-Y.C. performed scope studies for the cyclobutyl system. H.S.P. developed the pyridone–sulfonamide ligands and performed scope studies on the benzylic arylation. D.Q.P. developed the acyl-sulfonamide ligands. D.A.S. performed the DFT studies. D.A.S. and J.-Q.Y. prepared the paper. J.-Q.Y. directed the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Manuel van Gemmeren and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Aryl iodide scope with quaternized cyclobutane alcohol 3q.
Reactions were run according to General Procedure B. The reported yields are for isolated and purified products.
Extended Data Fig. 2 Poorly performing aryl iodides and alcohol substrates.
Due to the extremely low yields or lack of detectable product formation in most of these reactions, we were unable to isolate analytically pure samples of products except where specifically noted A) Poorly performing aryl iodides. Reactions were run according to General Procedure B with modifications as indicated. Aryl iodide screening was performed using either alcohol 3q (for 1-chloro-2-iodobenzene and 1-bromo-2-iodobenzene) or alcohol 3a (for the remaining aryl iodides) as the substrate. B) Poorly performing alcohol substrates. Alcohol screening was performed using methyl-4-iodobenzoate as the coupling partner. Bolded bonds indicate relative stereochemistry. C) Products isolated in low yields from reactions run with linear alcohols. §12 mol% L27 used. &Reaction run at 0.1 M. #Reactions were run according to General Procedure A using L24 as the ligand. ^Based on crude NMR vs. CH2Br2 internal standard. *Reaction run using 0.1 mmol of methyl-4-iodobenzoate as the limiting reagent (0.1 M in DCE) with 10 equivalents of the alcohol substrate.
Extended Data Fig. 3 Logic of double mutant cycle experiments.
Double mutant cycles are a classic tool used to quantify the energy of a non-covalent interaction of interest in a complex setting, including enzyme-substrate binding and fully synthetic systems such as molecular balances and zippers50. The interaction under investigation is perturbed by removing each interacting partner (here the HBD and HBA) both individually (affording a pair of single mutants) and simultaneously (to give the double mutant). Each of these modifications will affect more than just the proposed H-bonding interaction, but the non-cooperative effects can be cancelled by adding and subtracting the energies of the four species using the equation in the figure above. The resulting energy will reflect differences in cooperative interactions between the two interacting partners across the four structures. Ideally, each mutation would fully knock out the interaction under investigation without creating any new interactions with the other partner, in which case the equation would directly report the (de)stabilization due to the interaction under investigation in the parent system. In practice, however, it can be difficult to design modifications which meet these criteria since there are many ways in which the mutated substituents could still interact cooperatively (e.g., other non-covalent interactions like dipole-dipole interactions or steric clash, or via through-bond effects, such as changes in the Lewis acidity of the metal center). Thus, double mutant cycles must be designed carefully to ensure that they provide information about the interaction of interest rather than other cooperative interactions. To address the potential for confounding factors, we have calculated four double mutant cycles each for TS-1, TS-δ-1, TS-cis-γ-1, and TS-trans-γ-1 (see Extended Data Fig. 4 and Figs. S78, S83, and S88 for details, discussion, and tabulated energies and Figs. 4e and S71–S77, S79–S82, and S84–S87 for the actual cycles) employing different mutations designed to have very different potential confounding factors. The qualitative agreement between these models provides strong support for significant stabilization by the proposed H-bonding interaction.
Extended Data Fig. 4 Summary of computational double mutant cycles examined for benzylic C–H activation.
A) The HBA can be knocked out by replacing the acyl sulfonamide with a carboxylate or an amidate. The amidate preserves the identity of the chelating atom, but may significantly alter the electronics at Pd, whereas the carboxylate is more similar to the acyl sulfonamide electronically but changes the atom bound to Pd. B) The HBD can be knocked out by replacing the directing group with a methyl ether or an alkoxide. The methyl ether preserves the charge and nature of the directing group, but significantly alters the steric properties of the directing group, whereas the alkoxide significantly alters the electronic character of the directing group, but presents a similar steric profile to the alcohol. C) Tabulated data for the four cycles generated by the pairwise combinations of the ligand and substrate mutations described above (the tabulated data is taken from the cycles shown in Figs. 4e and S71–S73). While there are modest quantitative differences in the interaction energy measured for each cycle, they are all in qualitative agreement on the stabilization afforded by the interaction between the alcohol and the sulfonamide. Since the four cycles were chosen to have different potential confounding factors, the qualitative agreement strongly suggests that the observed stabilization is due at least in large part to the proposed H-bonding interaction.
Supplementary information
Supplementary Information
Hydrogen-bond-acceptor ligands enable distal C(sp3)–H arylation of free alcohols
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Strassfeld, D.A., Chen, CY., Park, H.S. et al. Hydrogen-bond-acceptor ligands enable distal C(sp3)–H arylation of free alcohols. Nature 622, 80–86 (2023). https://doi.org/10.1038/s41586-023-06485-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06485-8
This article is cited by
-
Ligand-modulated nickel-catalyzed regioselective silylalkylation of alkenes
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.