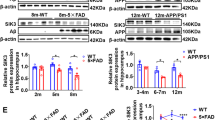
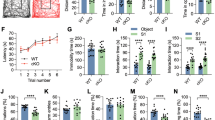
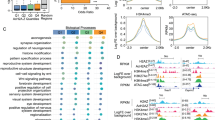
Abstract
Chromatin modifications, especially histone-tail acetylation, have been implicated in memory formation. Increased histone-tail acetylation induced by inhibitors of histone deacetylases (HDACis) facilitates learning and memory in wild-type mice as well as in mouse models of neurodegeneration. Harnessing the therapeutic potential of HDACis requires knowledge of the specific HDAC family member(s) linked to cognitive enhancement. Here we show that neuron-specific overexpression of HDAC2, but not that of HDAC1, decreased dendritic spine density, synapse number, synaptic plasticity and memory formation. Conversely, Hdac2 deficiency resulted in increased synapse number and memory facilitation, similar to chronic treatment with HDACis in mice. Notably, reduced synapse number and learning impairment of HDAC2-overexpressing mice were ameliorated by chronic treatment with HDACis. Correspondingly, treatment with HDACis failed to further facilitate memory formation in Hdac2-deficient mice. Furthermore, analysis of promoter occupancy revealed an association of HDAC2 with the promoters of genes implicated in synaptic plasticity and memory formation. Taken together, our results suggest that HDAC2 functions in modulating synaptic plasticity and long-lasting changes of neural circuits, which in turn negatively regulates learning and memory. These observations encourage the development and testing of HDAC2-selective inhibitors for human diseases associated with memory impairment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
West, A. E. et al. Calcium regulation of neuronal gene expression. Proc. Natl Acad. Sci. USA 98, 11024–11031 (2001)
Guan, Z. et al. Integration of long-term-memory-related synaptic plasticity involves bidirectional regulation of gene expression and chromatin structure. Cell 111, 483–493 (2002)
Kurdistani, S. K. & Grunstein, M. Histone acetylation and deacetylation in yeast. Nature Rev. Mol. Cell Biol. 4, 276–284 (2003)
Goldberg, A. D., Allis, C. D. & Bernstein, E. Epigenetics: a landscape takes shape. Cell 128, 635–638 (2007)
Alarcon, J. M. et al. Chromatin acetylation, memory, and LTP are impaired in CBP+/- mice: a model for the cognitive deficit in Rubinstein–Taybi syndrome and its amelioration. Neuron 42, 947–959 (2004)
Korzus, E., Rosenfeld, M. G. & Mayford, M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron 42, 961–972 (2004)
Levenson, J. M. et al. Regulation of histone acetylation during memory formation in the hippocampus. J. Biol. Chem. 279, 40545–40559 (2004)
Kumar, A. et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron 48, 303–314 (2005)
Vecsey, C. G. et al. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J. Neurosci. 27, 6128–6140 (2007)
Fischer, A. et al. Recovery of learning and memory is associated with chromatin remodelling. Nature 447, 178–182 (2007)
Bruserud, O. et al. Histone deacetylase inhibitors in cancer treatment: a review of the clinical toxicity and the modulation of gene expression in cancer cell. Curr. Pharm. Biotechnol. 8, 388–400 (2007)
Tsankova, N. M. et al. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nature Neurosci. 9, 519–525 (2006)
Renthal, W. et al. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron 56, 517–529 (2007)
Hockly, E. et al. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, ameliorates motor deficits in a mouse model of Huntington’s disease. Proc. Natl Acad. Sci. USA 100, 2041–2046 (2003)
Ferrante, R. J. et al. Histone deacetylase inhibition by sodium butyrate chemotherapy ameliorates the neurodegenerative phenotype in Huntington’s disease mice. J. Neurosci. 23, 9418–9427 (2003)
Witter, D. J. et al. Optimization of biaryl selective HDAC1&2 inhibitors (SHI-1:2). Bioorg. Med. Chem. Lett. 18, 726–731 (2008)
Guardiola, A. R. & Yao, T. P. Molecular cloning and characterization of a novel histone deacetylase HDAC10. J. Biol. Chem. 277, 3350–3356 (2002)
Hubbert, C. et al. HDAC6 is a microtubule-associated deacetylase. Nature 417, 455–458 (2002)
Salisbury, C. M. & Cravatt, B. F. Activity-based probes for proteomic profiling of histone deacetylase complexes. Proc. Natl Acad. Sci. USA 104, 1171–1176 (2007)
Roberson, E. D. et al. Reducing endogenous tau ameliorates amyloid β-induced deficits in an Alzheimer’s disease mouse model. Science 316, 750–754 (2007)
Luikenhuis, S., Giacometti, E., Beard, C. F. & Jaenisch, R. Expression of MeCP2 in postmitotic neurons rescues Rett syndrome in mice. Proc. Natl Acad. Sci. USA 101, 6033–6038 (2004)
Martin, S. J., Grimwood, P. D. & Morris, R. G. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu. Rev. Neurosci. 23, 649–711 (2000)
Finkbeiner, S. et al. CREB: a major mediator of neuronal neurotrophin responses. Neuron 19, 1031–1047 (1997)
Lunyak, V. V. et al. Corepressor-dependent silencing of chromosomal regions encoding neuronal genes. Science 298, 1747–1752 (2002)
Ballas, N. et al. REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell 121, 645–657 (2005)
Nott, A. et al. S-nitrosylation of histone deacetylase 2 induces chromatin remodelling in neurons. Nature 455, 411–415 (2008)
Hassig, C. A. et al. A role for histone deacetylase activity in HDAC1-mediated transcriptional repression. Proc. Natl Acad. Sci. USA 95, 3519–3524 (1998)
Montgomery, R. L. et al. Histone deacetylases 1 and 2 redundantly regulate cardiac morphogenesis, growth, and contractility. Genes Dev. 21, 1790–1802 (2007)
Kim, D. et al. Deregulation of HDAC1 by p25/Cdk5 in neurotoxicity. Neuron 60, 803–817 (2008)
Radulovic, J., Ruhmann, A., Liepold, T. & Spiess, J. Modulation of learning and anxiety by corticotropin-releasing factor (CRF) and stress: differential roles of CRF receptors 1 and 2. J. Neurosci. 19, 5016–5025 (1999)
Morris, R. G., Garrud, P., Rawlins, J. N. & O’Keefe, J. Place navigation impaired in rats with hippocampal lesions. Nature 297, 681–683 (1982)
Breslow, R., Marks, P., Rifkind, R. & Jursic, B. Novel Potent Inducers of Terminal Differentiation and Methods Thereof. US Patent WO/1993/007148 (1993)
Ramon-Molinier, E. Contemporary Research Methods in Neuroanatomy Synaptic Plasticity: Multiple Forms and Mechanisms (Springer, 1970)
Guan, J. S. et al. Interaction with vesicle luminal protachykinin regulates surface expression of δ-opioid receptors and opioid analgesia. Cell 122, 619–631 (2005)
Hernandez, A. I. et al. Protein kinase Mζ synthesis from a brain mRNA encoding an independent protein kinase Cζ catalytic domain. Implications for the molecular mechanism of memory. J. Biol. Chem. 278, 40305–40316 (2003)
Acknowledgements
We thank E. Scolnick, D. Fass, P. Sklar, T. Petryshen, B. A. Samuels, A. Fischer, C. Frank, D. Kim, S. Su and Y. Hayashi for advice and critical reading of the manuscript; T. Petryshen, A. Graybiel, J. Crittenden and M. C. Lewis for providing the T-maze behaviour model; R. Neve for providing tdTomato HSV. Funding was provided by a grant from the National Institute of Neurological Disorders and Stroke (2 ROI NS051874) to L.-H.T., by a research fund from the Stanley Center for Psychiatric Research to L.-H.T. and S.J.H., by the National Alliance for Research on Schizophrenia and Depression Foundation to S.J.H.; by a fellowship from the Damon-Runyon Cancer Research Foundation and The Dutch Cancer Society (KWF) to J.H.D. R.J. is supported by NIH grants (5-RO1-CA087869, 5-R37-CA084198, 5-RO1-HD0445022); R.A.D. is supported by the Robert A. and Renee E. Belfer Institute for Applied Cancer Science. L.-H.T. is an investigator of the Howard Hughes Medical Institute.
Author Contributions L.-H.T. designed, directed and coordinated the project. J.-S.G. designed and performed the behaviour tests, biochemical assays and morphological analysis in HDACi-treated animals and genetically modified animal models. S.J.H., R.M., J.E.B. contributed to the generation and characterization of HDACis. E.G. generated HDAC1/2OE mice in R.J.’s laboratory. J.-H.D. generated HDAC2KO mice in R.A.D.’s laboratory. N.J., W.X.Y., Y.Z. and E.G. contributed to behavioural tests and biochemical analysis. J.G. performed electrophysiological analysis. T.J.F.N. performed imaging assays for cultured neurons. R.A.D., J.-H.D., E.G. and R.J. critically reviewed the experimental data. The manuscript was written by J.-S.G., S.J.H. and L.-H.T. and was commented on by all the authors.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains Supplementary Data, Supplementary Table 1, Supplementary Figures 1-17 with Legends and Supplementary References. (PDF 7804 kb)
Rights and permissions
About this article
Cite this article
Guan, JS., Haggarty, S., Giacometti, E. et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature 459, 55–60 (2009). https://doi.org/10.1038/nature07925
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature07925
This article is cited by
-
Histone deacetylase inhibitors VPA and WT161 ameliorate the pathological features and cognitive impairments of the APP/PS1 Alzheimer’s disease mouse model by regulating the expression of APP secretases
Alzheimer's Research & Therapy (2024)
-
Dysregulation of histone deacetylases in ocular diseases
Archives of Pharmacal Research (2024)
-
Suv39h1 Silencing Recovers Memory Decline in Scopolamine-Induced Amnesic Mouse Model
Molecular Neurobiology (2024)
-
Alzheimer’s Disease-Related Epigenetic Changes: Novel Therapeutic Targets
Molecular Neurobiology (2024)
-
Shared genetic risk loci between Alzheimer’s disease and related dementias, Parkinson’s disease, and amyotrophic lateral sclerosis
Alzheimer's Research & Therapy (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.