Abstract
Although the function of zinc finger and BTB domain containing 16 (ZBTB16) in spermatogenesis is well documented, expression of ZBTB16 in germ cell tumors has not yet been studied. The aim of this study was to investigate the immunohistochemical expression and diagnostic utility of ZBTB16 in germ cell tumors. A total of 67 adult germ cell tumors were studied (62 testicular germ cell tumors, 2 ovarian yolk sac tumors, 1 mediastinal yolk sac tumor, and 2 retroperitoneal metastatic yolk sac tumors). The 62 testicular primary germ cell tumors are as follows: 34 pure germ cell tumors (20 seminomas, 8 embryonal carcinomas, 2 teratomas, 1 choriocarcinoma, 1 carcinoid, and 2 spermatocytic tumors) and 28 mixed germ cell tumors (composed of 13 embryonal carcinomas, 15 yolk sac tumors, 15 teratomas, 7 seminomas, and 3 choriocarcinomas in various combinations). Thirty-five cases contained germ cell neoplasia in situ. Yolk sac tumor was consistently reactive for ZBTB16. Among the 15 testicular yolk sac tumors in mixed germ cell tumors, all displayed moderate to diffuse ZBTB16 staining. ZBTB16 reactivity was present regardless of the histologic patterns of yolk sac tumor and ZBTB16 was able to pick up small foci of yolk sac tumor intermixed/embedded in other germ cell tumor subtype elements. Diffuse ZBTB16 immunoreactivity was also observed in 2/2 metastatic yolk sac tumors, 1/1 mediastinal yolk sac tumor, 2/2 ovarian yolk sac tumors, 2/2 spermatocytic tumors, 1/1 carcinoid, and the spermatogonial cells. All the other non-yolk sac germ cell tumors were nonreactive, including seminoma (n=27), embryonal carcinoma (n=21), teratoma (n=17), choriocarcinoma (n=4), and germ cell neoplasia in situ (n=35). The sensitivity and specificity of ZBTB16 in detecting yolk sac tumor among the germ cell tumors was 100% (20/20) and 96% (66/69), respectively. In conclusion, ZBTB16 is a highly sensitive and specific marker for yolk sac tumor.
Similar content being viewed by others
Main
Germ cell tumors account for >90% of the testicular neoplasms. Although most are malignant, testicular germ cell tumors are curable with appropriate treatment.1 Germ cell tumor can be morphologically subclassified into different subtypes, which differ in their clinical behavior as well as management.1, 2 Accurate histologic diagnosis and appropriate classification of testicular germ cell tumors is therefore critical. The diagnosis of testicular germ cell tumor is usually straightforward in the hands of experienced pathologists with routine hematoxylin-and-eosin-stained sections; however, different germ cell tumor subtype elements can have overlapping morphologies, and in mixed germ cell tumor, which comprises approximately half of the testicular germ cell tumor cases,2, 3, 4 different germ cell tumor subtypes can be intimately intermixed. This can make the distinction between individual germ cell tumor subtypes challenging.3, 4, 5, 6 Among all the germ cell tumor subtypes, yolk sac tumor, which has various histologic patterns, has been known to be the most commonly overlooked and underdiagnosed element3, 4, 5, 6, 7, 8 and ancillary diagnostic markers are frequently needed to facilitate its diagnosis. Earlier germ cell tumor biomarkers including placental-like alkaline phosphatase and α-fetoprotein, although helpful, show only moderate sensitivity and specificity.2, 9, 10, 11, 12 New germ cell markers such as NANOG, SOX2 and Sal-like protein 4 (SALL4) have also been identified; however, these markers lack specificity for individual germ cell tumor subtypes.2, 13, 14, 15, 16 In addition, their expression may be lost in metastases or after treatment.17, 18 Glypican-3 is a more recently identified sensitive marker for yolk sac tumor;19, 20 nevertheless, glypican-3 has also been reported in embryonal carcinoma, choriocarcinoma and teratoma.18, 19, 20
Zinc finger and BTB domain containing 16 (ZBTB16), first identified in a patient with acute promyelocytic leukemia, is a zinc finger transcription factor belonging to the POZ-Krüppel family that binds to specific DNA sequences with its C-terminal zinc fingers and suppresses transcription by recruiting co-repressors with its aminoterminal POZ domain.21, 22 ZBTB16 affects diverse signaling pathways including cell cycle, differentiation, and programmed cell death pathways in hematopoietic cells and in solid tumors as well,22, 23, 24, 25 and is involved in major developmental and biological processes such as spermatogenesis and stem cell maintenance, hind limb formation, hematopoiesis, immune regulation, and oncogenesis.21, 22, 26, 27
In the testes, ZBTB16 is expressed specifically in undifferentiated spermatogonia.21, 28 Studies have implied that ZBTB16 serves to promote spermatogonial stem cell self-renewal21, 28 and has a key role in the maintenance of normal spermatogenesis. Infertility is observed in Zbtb16-null mice due to the impairment of spermatogonial stem cell self-renewal and subsequent exhaustion of spermatogonia.28, 29 Although the function of ZBTB16 in spermatogenesis is relatively well studied, expression of ZBTB16 in germ cell tumor has not yet been reported. The aim of this study was to investigate the immunohistochemical detection of ZBTB16 in testicular cells with particular focus on its diagnostic utility in germ cell tumor.
Material and methods
Tissue Samples
Archived formalin-fixed and paraffin-embedded tissue blocks from 67 adult patients with germ cell tumors accessioned between January 2009 and January 2015 were obtained from the surgical pathology files of the University of Rochester Medical Center. This study was performed after approval by the institutional review board of the University of Rochester. Among the 67 germ cell tumors, 62 were testicular primary germ cell tumors, 2 were ovarian yolk sac tumors, and 1 was mediastinal yolk sac tumor. There were 2 cases of metastatic testicular germ cell tumors in the retroperitonum, 1 of which recurred after chemotherapy and contained 60% yolk sac tumor and 40% teratoma, and the other containing yolk sac tumor only. Of the 62 testicular primary germ cell tumors, 34 were pure germ cell tumors and 28 were mixed germ cell tumors. Among the 34 pure germ cell tumors, 20 were pure seminomas, 8 pure embryonal carcinomas, 2 pure teratomas, 1 pure choriocarcinoma, 1 monodermal carcinoid tumor, and 2 spermatocytic tumors. The 28 mixed germ cell tumors were composed of 13 embryonal carcinomas, 15 yolk sac tumors, 15 teratomas, 7 seminomas, and 3 choriocarcinomas in various combinations. Germ cell neoplasia in situ was noted in 35 of the 62 testicular germ cell tumor cases. Spermatogonia with various degrees of spermatogenesis were morphologically seen in the non-neoplastic seminiferous tubules in 41 cases, atrophic seminiferous tubules with prominent Sertoli cells were present in 33 cases, and interstitial Leydig cells were observed in 24 cases. There were also testicular appendages in some of the samples including epididymis (12 cases) and rete testis (9 cases).
Immunohistochemistry
Following deparaffinization and rehydration, charged slides with 5-μm-thick sections of tissue were treated with 3% hydrogen peroxide (H2O2), to eliminate endogenous peroxidase activity, and then processed for antigen retrieval with 10-mM citrate buffer pH 6.0 using a pressure cooker (Pascal; Dako Cytomation, Glostrup, Denmark) for 1 min at 125 °C, followed by slow cooling. The rest of the procedure was done in a DAKO automated instrument. All sections were rinsed with phosphate-buffered saline (137 mM NaCl, 2.7 mM potassium chloride, 4.2 mM sodium phosphate, and 1.5 mM potassium phosphate) and reacted with mouse anti-ZBTB16 antibody (D-9; sc-28319, Santa Cruz Biotechnology, Santa Cruz, CA) for 1.5 h at 1:500 dilution in phosphate-buffered saline containing 1% bovine serum albumin and 5% normal goat serum at room temperature. The sections were then incubated for 20 min with EnVision+ System horseradish peroxidase-labeled polymer conjugated with biotinylated anti-mouse secondary antibody and 3,3′-diaminobenzidine substrate. All slides were counterstained with hematoxylin, dehydrated, and cover slipped.
Analysis of Immunohistochemical Staining
Testicular cells, including cells from germ cell tumors, non-neoplastic germ cells, stroma cells and cells of testicular appendages were analysed for ZBTB16 immunoreactivity in a semi-quantitative way. Each histologic component of the mixed germ cell tumor was independently evaluated. Only nuclear staining of ZBTB16 was considered positive. Based on the extent of the immunoreactivity, the staining was graded as: virtually no (<1%) cell staining (negative), 1–25% of cell staining (focal), 25–50% of cell staining (moderate extent) and >50% of cell staining (diffuse). ZBTB16-positive spermatogonial cells and ZBTB16-negative Sertoli cells and Leydig cells when present were used as internal positive and negative control, respectively (see Results section for detail).
Results
Expression of ZBTB16 in Non-Neoplastic Testicular Cells
Germ cells
Primary spermatogonia that are located along the basement membrane of non-neoplastic seminiferous tubules were uniformly and strongly reactive with ZBTB16 in all the cases (n=41) (Figure 1a and b). The spermatogonia in the seminiferous tubules with either normal spermatogenesis or impaired spermatogenesis showed similar ZBTB16 positivity.
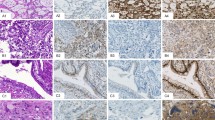
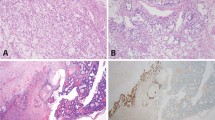
Hematoxylin–eosin stain and immunohistochemical expression of ZBTB16 in normal seminiferous tubules (NST), germ cell neoplasia in situ (GCNIS), spermatocytic tumor and testicular carcinoid. (a and b) Spermatogonia (arrowhead) are highlighted by strong ZBTB16 immunoreactivity. GCNISs (arrow) are non-immunoreactive with anti-ZBTB16. Spermatocytic tumor (c and d) and carcinoid (e and f) are strongly reactive with anti-ZBTB16.
Sex cord stroma cells
Although occasional cytoplasmic staining was noted in the Leydig cells, the nuclei of both Sertoli cells (n=33 cases) and Leydig cells (n=24 cases) were uniformly negative for ZBTB16.
Testicular appendages
Epididymis (n=12 cases) and rete testis (n=9 cases) were nonreactive with ZBTB16. The results of ZBTB16 immunohistochemical expression in the non-neoplastic testicular cells are summarized in Table 1.
Expression of ZBTB16 in Germ Cell Neoplasia In Situ
None of the germ cell neoplasia in situ were immunoreactive with ZBTB16 (n=35) (Figure 1a and b).
Expression of ZBTB16 in Germ Cell Tumors
Seminoma
All the seminoma (n=27) were completely negative for ZBTB16.
Embryonal carcinoma, teratoma, and choriocarcinoma
All the embryonal carcinoma (n=21) were nonreactive with ZBTB16. The mesoderm, ectoderm, and endoderm elements of teratoma (n=17) were all negative for ZBTB16. Although respiratory mucosa displayed cytoplasmic ZBTB16 staining, no nuclear staining was observed. No ZBTB16 was expressed in choriocarcinoma (n=4).
Spermatocytic tumor and testicular carcinoid tumor
Two spermatocytic tumors displayed diffuse immunoreactivity with ZBTB16. Among the three types of tumor cells, ZBTB16 reacted intensely with the large tumor cells and moderately with the intermediate tumor cells; the small tumor cells were nonreactive to weakly reactive (Figure 1c and d). Carcinoid (n=1), although thought to be a type of mondermal teratoma, was diffusely positive for ZBTB16 (Figure 1e and f).
Yolk sac tumor
The primary testicular yolk sac tumors in this study were all present as a component of a mixed germ cell tumor. The amount of yolk sac tumor in the mixed germ cell tumor ranged from 5% to 70%. Yolk sac tumor elements were frequently intermixed/embedded in the other accompanying subtypes of germ cell tumor components. Regardless of its relationship to other germ cell tumor components (demarcated or intermixed), yolk sac tumor was consistently reactive with ZBTB16. Among the 15 testicular yolk sac tumors in the mixed germ cell tumors, 10 (67%) had diffuse ZBTB16 staining, 5 (33%) had moderate extent of ZBTB16 staining, and none had <25% of tumor cells staining. ZBTB16 reactivity was present in all the histological patterns of yolk sac tumor (Figure 2a–h). Microcystic/myxoid/reticular patterns appeared to have the greatest percentage of ZBTB16 staining cells compared with the glandular and papillary patterns. In addition, in mixed germ cell tumors, ZBTB16 immunostaining was able to pick up small foci of yolk sac tumor that were embedded among the other germ cell tumor elements (mainly embryonal carcinoma and teratoma) (Figure 3a–d), in particular yolk sac tumors with reticular/microcystic/myxoid patterns, which might otherwise be mistaken as reactive stroma of embryonal carcinoma or mesenchymal elements of teratoma. The yolk sac tumor was also diffusely reactive with ZBTB16 in the two retroperitoneal metastatic yolk sac tumors (Figure 3e and f), two ovarian yolk sac tumors, and one mediastinal yolk sac tumor (Figure 3g and h).
Hematoxylin–eosin stain and immunohistochemical expression of ZBTB16 in yolk sac tumor embedded in embryonal carcinoma (a and b) and teratoma (c and d) is highlighted by ZBTB16 expression. Metastatic yolk sac tumor (e and f) and mediastinal yolk sac tumor (g and h) are strongly reactive with anti-ZBTB16.
The sensitivity of ZBTB16 in detecting yolk sac tumor (gonadal, extragonadal and metastatic) in this study was 100% (20/20). If spermatocytic tumor and carcinoid, both of which are extremely rare testicular tumors and are less likely to be morphologically confused with yolk sac tumor, were excluded, the specificity of ZBTB16 immunostaining for yolk sac tumor among the germ cell tumors was 100% (66/66). If these 2 rare entities were included, the specificity of ZBTB16 for yolk sac tumor was 96% (66/69)
Table 2 summarizes the results of ZBTB16 immunostaining in germ cell tumor and germ cell neoplasia in situ.
Discussion
Germ cell tumors often exhibit diverse morphologies and correct pathologic subclassification of germ cell tumor can, at times, be difficult. In particular, yolk sac tumor has a variety of growth patterns, which can be misinterpreted and mistaken for other germ cell tumor subtypes.2, 3, 4, 5, 6, 7, 8 In addition, primary yolk sac tumor often grows in intermixture with embryonal carcinoma and teratoma in mixed germ cell tumor.
The variable histological appearance, intermixture with other germ cell tumor subtypes, and its focal nature have made yolk sac tumor one of the most commonly undiagnosed germ cell tumor elements on routine hematoxylin and eosin sections.3, 4, 5, 6, 7, 8 Discrimination between yolk sac tumor and embryonal carcinoma is important, as an increased proportion of embryonal carcinoma is suggested to be associated with a worse prognosis.30, 31, 32 Yolk sac tumor can mimic embryonal carcinoma in both growth patterns and cytomorphology. In addition, yolk sac tumor and embryonal carcinoma can be intermixed, which may obscure small foci of embedded yolk sac tumor.3, 4, 5, 6, 7, 8 Metastatic mature teratoma is usually treated by surgery alone, whereas the presence of yolk sac tumor requires additional neoadjuvant therapy.33, 34, 35 Proliferative or hyperplastic endodermal glandular epithelium of teratoma or rare somatic adenocarcinoma arising from teratoma can mimic glandular yolk sac tumor.3, 4, 5, 6, 7, 8 Myxoid/reticular/microcystic yolk sac tumor may also be mistaken as edematous mesodermal teratomatous element or embryonal carcinoma-associated stroma, in particular when yolk sac tumor and embryonal carcinoma/teratoma are intimately associated. Yolk sac tumor mixed with seminoma in a primary tumor has different prognosis and is treated differently from pure seminoma.31 Solid yolk sac tumor can sometimes mimic seminoma.7 Seminoma can occasionally present with pseudoglandular or pseudocystic patterns, which may also be simulated by glandular or cystic yolk sac tumor.6 Owing to these morphologic challenges, biomarkers that can efficiently distinguish yolk sac tumor from other germ cell tumor elements would therefore be greatly helpful.
α-Fetoprotein is a traditional marker for yolk sac tumor; however, its sensitivity is limited and the staining is frequently patchy,18 with positivity being reported in ~60% of yolk sac tumor cases.18 α-Fetoprotein has also been reported to stain embryonal carcinoma and teratoma.36, 37, 38 In addition, α-fetoprotein may be lost in metastases or after treatment even if the primary tumor is positive for α-fetoprotein.17, 18
Glypican-3 is the other germ cell tumor marker that was recently identified to be valuable for diagnosis of yolk sac tumor. It has been reported that glypican-3 is more sensitive than α-fetoprotein,18 but might not be as specific as α-fetoprotein for yolk sac tumor. Glypican-3 can be positive in embryonal carcinoma, choriocarcinoma, and teratoma, as well as in some non-germ cell tumors.19, 39, 40, 41, 42
ZBTB16, a transcription repressor, has been known to have a key role in normal spermatogenesis.43 Its contribution to the pathogenesis of germ cell tumor and clinical value in the diagnosis of germ cell tumor, however, has not yet been investigated. In this study, we demonstrated that ZBTB16 was highly sensitive and specific for yolk sac tumor. ZBTB16 efficiently stained all the growth patterns of yolk sac tumor, including reticular, cystic, myxoid, glandular, papillary, polyvesicular, enteric, and solid patterns. ZBTB16 also picked up small foci of yolk sac tumor that were intermixed/embedded in other germ cell tumor subtype elements in mixed germ cell tumor, especially embryonal carcinoma and teratoma. ZBTB16 positivity was present in every yolk sac tumor case. ZBTB16 staining was consistently negative in non-yolk sac tumor germ cell tumors, except the rare spermatocytic tumor and carcinoid. All these indicate that ZBTB16 has higher sensitivity and specificity for yolk sac tumor.
Except carcinoid and prostatic adenocarcinoma,25, 44 ZBTB16 nuclear expression is barely seen in other tumors, including those from respiratory, gastrointestinal and biliary tracts, pancreas, kidney, bladder, and female genital tract.45 Despite only a few cases studied, the strong reactivity of anti-ZBTB16 with ovarian yolk sac tumor, metastatic yolk sac tumor, as well as extragonadal (mediastinal) yolk sac tumor also confirmed its potential value in distinguishing extra-testicular and metastatic yolk sac tumor from its other tumor mimics.
Strong ZBTB16 positivity in spermatocytic tumor is also useful in confirming this rare testicular tumor and can be used to differentiate it from seminoma, its major mimic.
Morphologically, both germ cell neoplasia in situ and spermatogonia present as enlarged germ cells. Although nuclear hyperchromasia, prominent nucleoli, as well as irregular nuclear contours can be used in distinguishing germ cell neoplasia in situ cells from spermatogonia, in certain circumstances, especially in the setting of suboptimal staining, the presence of artifact, small biopsy, and atrophic seminiferous tubules, it is not uncommon to mistake spermatogonia for germ cell neoplasia in situ cells. The consistently strong ZBTB16 stain in the spermatogonia of seminiferous tubules is helpful in distinguishing germ cell neoplasia in situ from atypical-appearing spermatogonia.
The other advantage of ZBTB16 is its nuclear staining. Unlike the cytoplasmic staining of α-fetoprotein and glypican-3, nuclear ZBTB16 immunostaining is less affected by hemorrhage, serum and secretions, or necrosis; therefore, the background staining is minimal.
In summary, our results indicate that ZBTB16 staining has excellent diagnostic utility for diagnosing yolk sac tumor. It is highly yolk sac tumor sensitive and specific, and the results are easy to interpret. Interestingly, ZBTB16 has been linked to the maintenance of adult germ cell stem cell populations through the modulation of SALL4.21 SALL4 is a recently discovered generic germ cell tumor marker, which is expressed in all the subtypes of germ cell tumor,16, 46 whereas, based on our results, ZBTB16 preferentially reacts with yolk sac tumor. Although interaction between SALL4 and ZBTB16 is important in germ cell development and maintenance of germ cells, the role of ZBTB16 and SALL4 in the development of germ cell tumors and the biological significance of their differential expression of ZBTB16 in yolk sac tumors among other germ cell tumors awaits further study.
References
Einhorn LH . Chemotherapeutic and surgical strategies for germ cell tumors. Chest Surg Clin N Am 2002;12:695–706.
Bahrami A, Ro JY, Ayala AG . An overview of testicular germ cell tumors. Arch Pathol Lab Med 2007;131:1267–1280.
Ye H, Ulbright TM . Difficult differential diagnoses in testicular pathology. Arch Pathol Lab Med 2012;136:435–446.
Ulbright TM . The most common, clinically significant misdiagnoses in testicular tumor pathology, and how to avoid them. Adv Anat Pathol 2008;15:18–27.
Ulbright TM . GCTs of the gonads: a selective review emphasizing problems in differential diagnosis, newly appreciated, and controversial issues. Mod Pathol 2005;18:S61–S79.
Ulbright TM, Young RH . Seminoma with tubular, microcystic and related patterns: a study of 28 cases of unusual morphologic variants that often cause confusion with yolk sac tumor. Am J Surg Pathol 2005;29:500–505.
Kao CS, Idrees MT, Young RH et al. Solid pattern yolk sac tumor: a morphologic and immunohistochemical study of 52 cases. Am J Surg Pathol 2012;36:360–367.
Magers MJ, Kao CS, Cole CD et al. “Somatic-type” malignancies arising from testicular germ cell tumors: a clinicopathologic study of 124 cases with emphasis on glandular tumors supporting frequent yolk sac tumor origin. Am J Surg Pathol 2014;38:1396–1409.
Iczkowski KA, Butler SL, Shanks JH et al. Trials of new germ cell immunohistochemical stains in 93 extragonadal and metastatic GCTs. Hum Pathol 2008;39:275–281.
Leroy X, Augusto D, Leteurtre E et al. CD30 and CD117 (c-kit) used in combination are useful for distinguishing embryonal carcinoma from seminoma. J Histochem Cytochem 2002;50:283–285.
Morinaga S, Ojima M, Sasano N . Human chorionic gonadotropin and alpha-fetoprotein in testicular GCTs. An immunohistochemical study in comparison with tissue concentrations. Cancer 1983;52:1281–1289.
Tickoo SK, Hutchinson B, Bacik J et al. Testicular seminoma: a clinicopathologic and immunohistochemical study of 105 cases with special reference to seminomas with atypical features. Int J Surg Pathol 2002;10:23–32.
Hart AH, Hartley L, Parker K et al. The pluripotency homeobox gene NANOG is expressed in human GCTs. Cancer 2005;104:2092–2098.
Jones TD, Ulbright TM, Eble JN et al. OCT4 staining in testicular tumors: a sensitive and specific marker for seminoma and embryonal carcinoma. Am J Surg Pathol 2004;28:935–940.
De Jong J, Stoop H, Gills AJ et al. Differential expression of SOX17 and SOX2 in germ cells and stem cells has biological and clinical implications. J Pathol 2008;215:21–30.
Cao D, Li J, Guo CC et al. SALL4 is a novel diagnostic marker for testicular germ cell tumors. Am J Surg Pathol 2009;33:1065–1077.
Mostofi FK . Histological change ostensibly induced by therapy in the metastasis of germ cell tumors of testis. Prog Clin Biol Res 1985;203:47–60.
Zynger DL, McCallum JC, Luan C et al. Glypican-3 has a higher sensitivity than alpha-fetoprotein for testicular and ovarian yolk sac tumour: immunohistochemical investigation with analysis of histological growth patterns. Histopathology 2010;56:750–757.
Preda O, Nicolae A, Aneiros-Fernández J et al. Glypican 3 is a sensitive, but not a specific, marker for the diagnosis of yolk sac tumours. Histopathology 2011;58:312–314.
Zynger DL, Dimov ND, Luan C et al. Glypican 3: a novel marker in testicular germ cell tumors. Am J Surg Pathol 2006;30:1570–1575.
Suliman BA, Xu D, Williams BR . The promyelocytic leukemia zinc finger protein: two decades of molecular oncology. Front Oncol 2012;2:74–96.
Kolesnichenko M, Vogt PK . Understanding PLZF: two transcriptional targets, REDD1 and smooth muscle α-actin, define new questions in growth control, senescence, self-renewal and tumor suppression. Cell Cycle 2011;10:771–775.
Brunner G, Reitz M, Schwipper V et al. Increased expression of the tumor suppressor PLZF is a continuous predictor of long-term survival in malignant melanoma patients. Cancer Biother Radiopharm 2008;23:451–459.
Cheung M, Pei J, Pei Y et al. The promyelocytic leukemia zinc-finger gene, PLZF, is frequently downregulated in malignant mesothelioma cells and contributes to cell survival. Oncogene 2010;29:1633–1640.
Xiao GQ, Unger P, Yang Q et al. Loss of PLZF expression in prostate cancer by immunohistochemistry correlates with tumor aggressiveness and metastasis. PLoS ONE 2015;10:e0121318.
Weinreich MA, Odumade OA, Jameson SC et al. T cells expressing the transcription factor PLZF regulate the development of memory-like CD8+ T cells. Nat Immunol 2010;11:709–716.
Wang X, Wang L, Guo S et al. Hypermethylation reduces expression of tumor-suppressor PLZF and regulates proliferation and apoptosis in non-small-cell lung cancers. FASEB J 2013;27:4194–4203.
Costoya JA, Hobbs RM, Barna M et al. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat Genet 2004;36:653–659.
Song HW, Wilkinson MF . Transcriptional control of spermatogonial maintenance and differentiation. Semin Cell Dev Biol 2014;30:14–26.
Rajpert-De Meyts E . Recent advances and future directions in research on testicular germ cell cancer. Int J Androl 2007;30:192–197.
Mickisch GH . Prognostic parameters for the management of advanced testis tumours. Curr Opin Urol 2000;10:465–471.
Moul JW, McCarthy WF, Fernandez EB et al. Percentage of embryonal carcinoma and of vascular invasion predicts pathological stage in clinical stage I nonseminomatous testicular cancer. Cancer Res 1994;54:362–364.
Sonneveld DJ, Sleijfer DT, Koops HS et al. Mature teratoma identified after postchemotherapy surgery in patients with disseminated nonseminomatous testicular germ cell tumors: a plea for an aggressive surgical approach. Cancer 1998;82:1343–1351.
Fizazi K, Tjulandin S, Salvioni R et al. Viable malignant cells after primary chemotherapy for disseminated nonseminomatous germ cell tumors: prognostic factors and role of postsurgery chemotherapy— results from an international study group. J Clin Oncol 2001;19:2647–2657.
Einhorn LH, Williams SD, Chamness A et al. High-dose chemotherapy and stem-cell rescue for metastatic germ-cell tumors. N Engl J Med 2007;357:340–348.
Fowler JE Jr, Sesterhenn I, Stutzman RE et al. Localization of alpha-fetoprotein and human chorionic gonadotropin to specific histologic types of nonseminomatous testicular cancer. Urology 1983;22:649–654.
Wittekind C, Wichmann T, Von Kleist S . Immunohistological localization of AFP and HCG in uniformly classified testis tumors. Anticancer Res 1983;3:327–330.
McCluggage WG, Young RH . Immunohistochemistry as a diagnostic aid in the evaluation of ovarian tumors. Semin Diagn Pathol 2005;22:3–32.
Zynger DL, Everton MJ, Dimov ND et al. Expression of glypican 3 in ovarian and extragonadal germ cell tumors. Am J Clin Pathol 2008;130:224–230.
Yamauchi N, Watanabe A, Hishinuma M et al. The glypican 3 oncofetal protein is a promising diagnostic marker for hepatocellular carcinoma. Mod Pathol 2005;18:1591–1598.
Saikali Z, Sinnett D . Expression of glypican 3 (GPC3) in embryonal tumors. Int J Cancer 2000;89:418–422.
Toretsky JA, Zitomersky NL, Eskenazi AE et al. Glypican-3 expression in Wilms tumor and hepatoblastoma. J Pediatr Hematol Oncol 2001;23:496–499.
Buaas FW, Kirsh AL, Sharma M et al. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet 2004;36:647–652.
Hechtman JF, Beasley MB, Kinoshita Y et al. Promyelocytic leukemia zinc finger and histone H1.5 differentially stain low- and high-grade pulmonary neuroendocrine tumors: a pilot immunohistochemical study. Hum Pathol 2013;44:1400–1405.
Straub S, Unger PD, Yang Q et al. Preferential expression of PLZF in benign prostatic epithelium and low grade prostate cancer. Mod Pathol 2015;28:261A.
Cao D, Humphrey PA, Allan RW . SALL4 is a novel sensitive and specific marker for metastatic germ cell tumors, with particular utility in detection of metastatic yolk sac tumors. Cancer 2009;115:2640–2651.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Xiao, GQ., Li, F., Unger, P. et al. ZBTB16: a novel sensitive and specific biomarker for yolk sac tumor. Mod Pathol 29, 591–598 (2016). https://doi.org/10.1038/modpathol.2016.46
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2016.46