Abstract
Hepatocyte Nuclear Factor 1 beta (HNF1β) is a transcription factor which plays an important role during early organogenesis, especially of the pancreato-biliary and urogenital tract. Furthermore, HNF1β is an established marker in the differential diagnosis of ovarian cancer and shows a distinct nuclear expression in the clear cell carcinoma subtype. Recently, it has been described in yolk sac tumor, which represents a common component in many non-seminomatous germ cell tumors. Due to its broad histologic diversity, the diagnosis may be challenging and additional tools are very helpful in the workup of germ cell tumors. Immunohistochemistry was used to study HNF1β expression in a tissue microarray (TMA) of 601 testicular germ cell tumors including seminoma, embryonal carcinoma, yolk sac tumor, choriocarcinoma, teratoma, germ cell neoplasia in situ (GCNIS), and normal tissue. The expression pattern was compared to glypican 3 (GPC3) and α-fetoprotein (AFP), two markers currently in use for the detection of yolk sac tumor. HNF1β showed a distinct nuclear staining in comparison to the cytoplasmic pattern of GPC3 and AFP. The sensitivity and specificity of HNF1β were 85.4% and 96.5%, of GPC3 83.3% and 90.7%, of AFP 62.5% and 97.7%. We conclude that HNF1β allows a reliable distinction of yolk sac tumor from other germ cell tumor components. Therefore, we propose HNF1β as a novel and robust marker in the immunohistochemical workup of testicular germ cell tumors.
Similar content being viewed by others
Introduction
Testicular germ cell tumors (GCT) represent only 1% of all cancers in males. They are however, the most common neoplasms of the testis. Their predominance in young men in the fertile and productive phase of life makes them an important entity [1]. WHO 2016 classification divided TGCT into two major groups according to whether they derived from germ cell neoplasia in situ (GCNIS) (postpubertal GCT) or not (spermatocytic tumors, prepubertal-type teratoma, and yolk sac tumor (YST)). Testicular GCT derived from GCNIS can further be classified into two main categories which have an impact on the clinical management: Seminomas and non-seminomatous GCT. The latter are subdivided into undifferentiated components (embryonal carcinoma) and into components with embryonal differentiation (teratoma) and extra-embryonal differentiation (choriocarcinoma and YST). Due to this heterogeneity, morphologic overlap between the different subtypes can be a challenge in daily practice. Nonetheless, the accurate histopathological diagnosis is critical for further patient management [2]. To enhance diagnostic accuracy, immunohistochemistry can serve as a valuable tool.
Among the mentioned GCT subtypes, YST shows the broadest morphological spectrum with more than ten described architectural patterns [3]. This diversity highlights the need for reliable markers. Current diagnostic algorithms recommend α-fetoprotein (AFP) and glypican 3 (GPC3) as YST markers, although their sensitivity and specificity are not perfect. Recently, Rougemont et al. reported on hepatocyte nuclear factor 1 beta (HNF1β) expression in 45 testicular and ovarian GCT and concluded that it is a sensitive and reliable marker for the detection of YST [4].
The aim of our study was to assess HNF1β expression in a large cohort of over 600 testicular GCT and to compare its sensitivity and specificity to the established markers AFP and GPC3.
Materials and methods
Patient selection
Paraffin blocks from 601 testicular GCT (period from 1990 to 2014, mean patient age of 36 years) were retrieved from the archives of Department of Pathology and Molecular Pathology, University Hospital Zurich, Switzerland. Two pathologists with special expertise in testicular pathology (AG, PKB) re-evaluated the slides and classified the tumors according to the 2016 WHO Classification. The resulting cohort consisted of 392 pure seminomas (65.2%), 147 non-seminomatous GCT (23.6%), 58 mixed seminomatous and non-seminomatous GCT (9.7%), and 4 spermatocytic tumors (0.7%).
Tissue microarray
A tissue microarray (TMA) was created as previously described [5]. Two tissue cores with a diameter of 0.6 mm were taken from every tumor. In mixed GCT every subtype was punched twice separately in order to reflect the tumor heterogeneity. In summary, the TMA contained the following components: 450 seminomas, 123 embryonal carcinomas, 48 YSTs, 42 teratomas, 8 choriocarcinomas, 4 spermatocytic tumors and 24 precursor lesions (GCNIS) from adjacent testicular tissue. In addition, nonneoplastic testicular tissue was included from 35 patients who underwent diagnostic procedures due to infertility. During processing 17 tissue cores were lost. In total, 1451 testicular tissue cores were analyzed.
Whole slides
In addition to the TMA, 15 whole slides of GCT with at least 20% of YST component were selected in order to analyze the immunohistochemical staining in different growth patterns. Nine cases were primary tumors, six cases metastases. The blocks chosen covered the most common growth patterns: microcystic-reticular, macrocystic, solid, glandular, and hepatoid. If a case showed different growth patterns, each pattern was analyzed separately. Details are summarized in Table 2.
Histology and immunohistochemistry
Three micro-thick sections of TMA blocks were mounted on glass slides (SuperFrost Plus; Menzel, Braunschweig, Germany), deparaffinized, rehydrated, and stained with hematoxylin and eosin using standard protocols.
We investigated the expression of HNF1β using a polyclonal antibody (SIGMA Chemical Company, dilution 1:200). The immunostaining was performed with the Leica Bond III 255 Stainer. Positive control tissues were normal kidney, liver, lung, prostate, and brain tissue. The staining pattern observed was nuclear.
GPC3 expression was investigated by using the antibody clone 1G12 (DCS Immuno Line, dilution 1:100). Paraffin embedded cell blocks of several cell lines were used as positive controls (Myeloid cell line, Marimo; human ovary adenocarcinoma: Ovcar-3; Human melanoma cell line: SK-Mel-30; Human hepatocellular carcinoma: HepG2; human colon adenocarcinoma: SW480). The staining pattern observed was both membranous and cytoplasmic.
For AFP immunostaining, a polyclonal antibody (DAKO, dilution 1:1000) was used. Fetal liver tissue was used as a positive control. The positive tissues often showed a granular cytoplasmic staining pattern. Sometimes a strong unspecific background staining, especially in necrotic and cystic areas was noted, as is characteristic for a secreted protein. This background reaction was not considered positive.
Both stainings (GPC3 and AFP) were performed on the Ventana Stainer platform in combination with OptiView DAB Kit. The microarray spots were digitalized and evaluated by two pathologists (AG, PKB) using imaging software (NanoZoomer by Hamamatsu). Tissue cores were dichotomized into positive vs negative cases. All cores with >5% of positive cells (according to the above mentioned staining patterns) were counted as positive. If one core of the punched tumor component was positive and the other one negative, the case was considered positive.
Results
HNF1β immunohistochemistry
Due to a moderate to strong nuclear staining the expression of HNF1β was easy to evaluate. Overall, the YST tumor component showed a homogeneous staining pattern in 31/48 cases (both cores positive) and a heterogeneous staining pattern in 10/48 cases (only one core positive). Of the 48 cases assessed, seven were found to be negative.
In the other non-seminomatous components a weak to moderate HNF1β expression was observed in 24/346 cases. All seminomas, spermatocytic tumors, GCNIS, and nonneoplastic testicular tissue samples were negative.
HNF1β exhibited a sensitivity of 85% (the 95% CI is 0.7162–0.9345) and a specificity of 96.5% (the 95% CI is 0.9476–0.9770).
GPC3 immunohistochemistry
In contrast to HNF1β, the staining pattern of GPC3 was membranous and/or cytoplasmic. Due to a weak background staining, only a moderate to strong signal was considered positive. A more or less homogeneous pattern was observed in 32/48 YST cases (both cores positive), whereas a heterogeneous pattern was seen in 8/48 cases (only one core positive). Of the 48 YST cases investigated, 8 were negative. Among non YST cases GPC3 expression was weak to moderate. It was most commonly detected in embryonal carcinoma (57/123 cases), 1/42 teratoma and 6/8 choriocarcinoma. Interestingly, 27/450 seminoma and 16/25 GCNIS showed a faint homogenous staining pattern which was interpreted as unspecific background and finally considered negative.
GPC3 showed a sensitivity of 83.3% (the 95% CI is 0.6923–0.9203) and a specificity of 90.7% (the 95% CI is 0.8818–0.9269).
AFP immunohistochemistry
The third marker AFP had a typically granular cytoplasmic expression. In our study, it showed a homogeneous staining pattern in 20/48 cases of YST (both cores positive) and a heterogeneous staining pattern in 10/48 cases (only one core positive). Of the YST investigated cases, 18/48 were negative. AFP was positive in 11/123 embryonal carcinoma, in 3/42 teratoma, and in 2/8 choriocarcinoma. Spermatocytic tumors, seminoma and GCNIS were negative.
The sensitivity of AFP was 62.5% (the 95% CI is 0.4733–0.7567) and the specificity was 97.7% (the 95% CI is 0.9616–0.9862).
All results are summarized in Table 1 and Fig. 1.
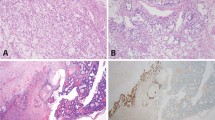
a Yolk sac tumor (YST). b Embryonal carcinoma (EC). c Teratoma (intestinal differentiation). d Choriocarcinoma (CC). GPC3 with a negative example of YST (A2). EC shows a weak cytoplasmic staining (B2). Teratoma is negative (C2). CC with scattered weakly stained syncytiotrophoblasts (D2). AFP with strong and diffuse positivity in YST (A3). EC (B3), Teratoma (C3) and CC (D3) are negative. HNF1β shows a strong nuclear expression in YST (A4). EC with no nuclear staining (B4). Glandular proliferations with intestinal differentiation in teratoma exhibit a striking nuclear staining (C4). CC with a diffuse cytoplasmic staining (D4). All images ×200 magnification.
Whole slides
A semiquantitative evaluation was performed: homogenous expression (>50% positive cells), heterogenous expression (5–50% positive cells), scattered single cells (<5% positive cells).
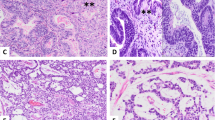
Strong and homogenous HNF1β expression was detected in all cases, except in solid YST in which a heterogenous expression pattern was observed. In contrast, GPC3 and AFP showed a much more heterogenous staining pattern, sometimes only with scattered positive cells. In one case (lymph node metastasis) GPC3 and AFP were negative. The results are summarized in Table 2 and Fig. 2.
Discussion
In this study of more than 600 testicular GCT, we analyzed HNF1β expression in testicular YST and non YST components and compared it to the commonly used and recommended markers AFP and GPC3 [6]. We found that HNF1β had a comparable sensitivity relative to GPC3 (85.4% versus 83.3%) and a comparable specificity relative to AFP (96.5% versus 97.7%). However, HNF1β had a higher sensitivity than AFP (85.4% versus 62.5%) and a slightly higher specificity than GPC3 (96.5% versus 90.7%).
Postpubertal testicular GCT can show a striking heterogeneity consisting of different components, e.g., seminoma, embryonal carcinoma, YST, choriocarcinoma, and teratoma. The correct diagnosis and quantification of the different components has an important influence on further therapeutic management [2]. The morphologic overlap between the distinct tumor subtypes can represent a significant diagnostic challenge [7, 8]. This is further compounded by the fact that YST alone is known for its broad morphology. The current WHO classification lists 11 different growth patterns: microcystic/reticular, myxomatous, macrocystic, solid, glandular/alveolar, endodermal sinus/perivascular, hepatoid, papillary, sarcomatoid/spindle cell, parietal and polyvescicular vitelline. All of them may occur in combination and can imitate other GCT [3]. In particular, the solid and glandular pattern can be difficult to differentiate from embryonal carcinoma, which behaves in a more aggressive fashion and may need a more intensive treatment [9]. Therefore, immunohistochemistry is recommended in difficult cases [6].
Currently, AFP and GPC3 are the best characterized YST markers [10]. During development, AFP is physiologically secreted by the yolk sac [11]. Up to now, AFP remains the gold standard marker for YST and correlates with corresponding serum levels. Hence, it is an integral part of clinical workup and follow-up in GCT patients [12]. AFP however, may not be the optimal marker as relevant serum AFP isoforms can also be detected in patients with nonneoplastic and neoplastic liver disease [13]. AFP immunohistochemistry shows a heterogeneous pattern with a granular cytoplasmic staining [10]. When compared to AFP, GPC3 has been shown to be a more sensitive marker for YST [14, 15], although the expression pattern was also found to be heterogeneous. Interestingly, similar to that observed for AFP, GPC3 can also be expressed in liver neoplasms [16], and in other non-testicular tumors [17,18,19].
HNF1β is a nuclear protein that is a member of the homeodomain-containing superfamily of transcription factors [20]. Its expression was previously observed in fetal liver, pancreas, stomach, lung, and kidney, indicating a possible role in the development of these organs [21, 22]. HNF1β mutations can cause renal cysts and maturity-onset diabetes of the young (MODY5) [23]. Rebouissou et al. suggested that HNF1β germline mutations predispose to renal tumors and proposed a role for HNF1β as a tumor suppressor in chromophobe renal cell carcinoma [24]. Moreover, a possible association between HNF1β polymorphisms and susceptibility to prostate cancer is described [25]. Kato et al. reported an overexpression of HNF1β not only in clear cell carcinoma of the ovary but also in endometriosis. In both entities, the authors observed a reduction of apoptosis suggesting a potential role for HNF1β in inhibition of apoptotic pathway activation [26]. In pathological practice, HNF1β is the current marker used for detection of clear cell carcinoma of the ovary [27, 28]. Interestingly, the somatic subtype of YST can arise in clear cell carcinoma of the ovary, both of which express HNF1β [29]. Based on this finding, Rougemont and Tille investigated HNF1β expression in 45 testicular and ovarian YST. In their study, the sensitivity was 100% and the specificity 80%. In summary, and in accordance with data presented here, they concluded that HNF1β represents a reliable YST marker [4].
In our study, we concentrated on the analysis of HNF1β, AFP, and GPC3 expression in testicular GCT to establish specificity and sensitivity values for each marker. We chose a TMA-based approach which allowed us to investigate more than 600 tumors. HNF1β expression was found in 85.4% of YST, compared to 83.3% for GPC3 and 62.5% for AFP. Specificity values were 96.5% (HNF1β), 90.7% (GPC3), and 97.7% (AFP). HNF1β expression was also detected in 16 cases of teratoma, 7 cases of embryonal carcinoma and in one case of choriocarcinoma (Table 1 and Fig. 1). The HNF1β-positivity in teratomas was only seen in mature and not in immature elements, particularly in glands with intestinal differentiation which is in line with previous findings by Rougemont and Tille [4]. In the cases of HNF1β positive embryonal carcinoma, only a few cells were positive. Interestingly, GPC3 immunostaining was also positive in these cases, suggesting small foci of previously undetected YST. The only HNF1β positive choriocarcinoma case exhibited a weak nuclear staining in a background cytoplasmic pattern which was in line with the results of Rougemont and Tille who observed the identical pattern in their cases of choriocarcinomas [4].
In contrast to HNF1β, GPC3 also stained 46.3% of embryonal carcinomas and 75% (6/8) of choriocarcinomas. In these cases, the intensity of staining was generally weak to moderate in comparison to YST, which exhibited a moderate to strong GPC3 staining. Hence, GPC3 does not reach the specificity of HNF1β or AFP. Similar results were also described by other groups [15, 30]. AFP showed a slightly higher specificity than HNF1β in our study (97.7% versus 96.5%) but the sensitivity was inferior (62.5% versus 85.4%). In addition, HNF1β expression is nuclear which makes it easier to evaluate than the cytoplasmic and membranous staining pattern seen in AFP and GPC3 immunohistochemistry. This facilitates the detection especially of very small foci of YST.
Remarkably, Rougemont and Tille described a sensitivity of 100% for HNF1β in their study but the immunostainings were conducted on whole slides. The reason for our lower sensitivity value might be due to the TMA approach, which may underestimate the real prevalence of positive cases because of sampling errors and tumor heterogeneity. Thus, we studied 15 additional GCT with a considerable percentage of YST on whole slides to analyze HNF1β heterogeneity in different YST growth patterns. In fact, in all cases (primary tumors and metastases) we could demonstrate a strong and quite homogeneous HNF1β expression except in solid YST, in which less than 50% tumor cells were positive. In contrast, AFP and GPC more often showed a heterogeneous pattern. However, the number of examined standard blocks is small and we could not analyze all reported YST growth patterns. Therefore, our study remains somewhat weakened by the TMA approach although it allowed a larger number of cases to be examined.
There exist some more limitations. Firstly, we mainly investigated untreated tumors. After chemotherapy, morphology can be much more difficult to interpret due to regressive changes and the persistence of unusual growth patterns [31, 32]. Moreover, protein expression can change after treatment. For instance, CD30 expression can be lost in embryonal carcinoma in a post-chemotherapeutic setting [33]. Secondly, our TMA cohort consisted of primary tumors. Sometimes testicular GCT primarily manifest or relapse with metastasis. In this setting, correct diagnosis may be challenging because of morphologic overlap to other tumor entities like carcinomas [34]. Thirdly, somatic-type malignancies arising in GCT were not included in our study. Thus, further investigations of HNF1β expression are needed to further elucidate the diagnostic utility of HNF1β in the three scenarios described.
In summary, we investigated HNF1β expression in more than 600 testicular GCT. Compared to the commonly used YST markers AFP and GPC3, HNF1β immunohistochemistry has a higher sensitivity than AFP and a higher specificity than GPC3. Furthermore, the nuclear expression pattern makes it easy to evaluate. Therefore, we conclude that HNF1β is a reliable marker in the diagnosis of YST and recommend that it be added to immunohistochemical panels in the differential diagnosis of testicular GCT.
References
Trabert B, Chen J, Devesa SS, Bray F, McGlynn KA. International patterns and trends in testicular cancer incidence, overall and by histologic subtype, 1973–2007. Andrology. 2015;3:4–12.
Honecker F, Aparicio J, Berney D, Beyer J, Bokemeyer C, Cathomas R, et al. ESMO Consensus Conference on testicular germ cell cancer: diagnosis, treatment and follow-up. Ann Oncol. 2018;29:1658–86.
Ulbright TM, Amin MB, Balzer B, Berney DM, Epstein JI, Guo C, et al. Tumours of the testis and paratesticular tissue, In: Moch H, Humphrey PA, Ulbright TM, Reuter VE, editors. WHO Classification of Tumours of the Urinary System and Male Genital Organs. Lyon: WHO Press; 2016.
Rougemont A-L, Tille J-C. Role of HNF1β in the differential diagnosis from other germ cell tumors. Hum Pathol. 2018;81:26–36.
Bode PK, Barghorn A, Fritzsche FR, Riener MO, Kristiansen G, Knuth A, et al. MAGEC2 is a sensitive and novel marker for seminoma: a tissue microarray analysis of 325 testicular germ cell tumors. Mod Pathol. 2011;24:829.
Ulbright TM, Tickoo SK, Berney DM, Srigley JR, Group IIiDUP. Best practices recommendations in the application of immunohistochemistry in testicular tumors: report from the International Society of Urological Pathology consensus conference. Am J Surg Pathol. 2014;38:e50–9.
Ulbright TM. Pitfalls in the interpretation of specimens from patients with testicular tumours, with an emphasis on variant morphologies. Pathology. 2018;50:88–99.
Rajab R, Berney DM. Ten testicular trapdoors. Histopathology. 2008;53:728–39.
Kao C-S, Idrees MT, Young RH, Ulbright TM. Solid pattern yolk sac tumor: a morphologic and immunohistochemical study of 52 cases. Am J Surg Pathol. 2012;36:360–7.
Nogales FF, Preda O, Nicolae A. Yolk sac tumours revisited. A review of their many faces and names. Histopathology. 2012;60:1023–33.
Mizejewski GJ. Alpha-fetoprotein structure and function: relevance to isoforms, epitopes, and conformational variants. Exp Biol Med. 2001;226:377–408.
Gilligan TD, Seidenfeld J, Basch EM, Einhorn LH, Fancher T, Smith DC, et al. American Society of Clinical Oncology Clinical Practice Guideline on uses of serum tumor markers in adult males with germ cell tumors. J Clin Oncol. 2010;28:3388–404.
Tsuchida Y, Kaneko M, Fukui M, Sakaguchi H, Ishiguro T. Three different types of alpha-fetoprotein in the diagnosis of malignant solid tumors: use of a sensitive lectin-affinity immunoelectrophoresis. J Pediatr Surg. 1989;24:350–5.
Zynger DL, Dimov ND, Luan C, Teh BT, Yang XJ. Glypican 3: a novel marker in testicular germ cell tumors. Am J Surg Pathol. 2006;30:1570–5.
Zynger DL, McCallum JC, Luan C, Chou PM, Yang XJ. Glypican 3 has a higher sensitivity than alpha-fetoprotein for testicular and ovarian yolk sac tumour: immunohistochemical investigation with analysis of histological growth patterns. Histopathology. 2010;56:750–7.
Yamauchi N, Watanabe A, Hishinuma M, Ohashi K, Midorikawa Y, Morishita Y, et al. The glypican 3 oncofetal protein is a promising diagnostic marker for hepatocellular carcinoma. Mod Pathol. 2005;18:1591–8.
Aviel-Ronen S, Lau SK, Pintilie M, Lau D, Liu N, Tsao MS, et al. Glypican-3 is overexpressed in lung squamous cell carcinoma, but not in adenocarcinoma. Mod Pathol. 2008;21:817–25.
Ou-Yang RJ, Hui P, Yang XJ, Zynger DL. Expression of glypican 3 in placental site trophoblastic tumor. Diagn Pathol. 2010;5:64.
Umezu T, Shibata K, Kajiyama H, Yamamoto E, Nawa A, Kikkawa F. Glypican-3 expression predicts poor clinical outcome of patients with early-stage clear cell carcinoma of the ovary. J Clin Pathol. 2010;63:962–6.
Bach I, Mattei M-G, Cereghini S, Yaniv M. Two members of an HNF1 homeoprotein family are expressed in human liver. Nucleic Acids Res. 1991;19:3553–9.
Barbacci E, Reber M, Ott MO, Breillat C, Huetz F, Cereghini S. Variant hepatocyte nuclear factor 1 is required for visceral endoderm specification. Development. 1999;126:4795–805.
Coffinier C, Barra J, Babinet C, Yaniv M. Expression of the vHNF1/HNF1beta homeoprotein gene during mouse organogenesis. Mech Dev. 1999;89:211–3.
El‐Khairi R, Vallier L. The role of hepatocyte nuclear factor 1β in disease and development. Diabetes, Obes Metab. 2016;18:23–32.
Rebouissou S, Vasiliu V, Thomas C, Bellanné-Chantelot C, Bui H, Chrétien Y, et al. Germline hepatocyte nuclear factor 1α and 1β mutations in renal cell carcinomas. Hum Mol Genet. 2005;14:603–14.
Thomas G, Jacobs KB, Yeager M, Kraft P, Wacholder S, Orr N, et al. Multiple loci identified in a genome-wide association study of prostate cancer. Nat Genet. 2008;40:310.
Kato N, Sasou S-i, Motoyama T. Expression of hepatocyte nuclear factor-1beta (HNF-1beta) in clear cell tumors and endometriosis of the ovary. Mod Pathol. 2006;19:83.
Tsuchiya A, Sakamoto M, Yasuda J, Chuma M, Ohta T, Ohki M, et al. Expression profiling in ovarian clear cell carcinoma: identification of hepatocyte nuclear factor-1 beta as a molecular marker and a possible molecular target for therapy of ovarian clear cell carcinoma. Am J Pathol. 2003;163:2503–12.
Fadare O, Zhao C, Khabele D, Parkash V, Quick CM, Gwin K, et al. Comparative analysis of Napsin A, alpha-methylacyl-coenzyme A racemase (AMACR, P504S), and hepatocyte nuclear factor 1 beta as diagnostic markers of ovarian clear cell carcinoma: an immunohistochemical study of 279 ovarian tumours. Pathology. 2015;47:105–11.
Nogales FF, Prat J, Schuldt M, Cruz-Viruel N1, Kaur B3, D’Angelo E, et al. Germ cell tumour growth patterns originating from clear cell carcinomas of the ovary and endometrium: a comparative immunohistochemical study favouring their origin from somatic stem cells. Histopathology. 2018;72:634–47.
Preda O, Nicolae A, Aneiros-Fernandez J, Borda A, Nogales FF. Glypican 3 is a sensitive, but not a specific, marker for the diagnosis of yolk sac tumours. Histopathology. 2011;58:312–4.
Berney DM, Lu YJ, Shamash J, Idrees M. Postchemotherapy changes in testicular germ cell tumours: biology and morphology. Histopathology. 2017;70:26–39.
Howitt BE, Magers MJ, Rice KR, Cole CD, Ulbright TM. Many postchemotherapy sarcomatous tumors in patients with testicular germ cell tumors are sarcomatoid yolk sac tumors: a study of 33 cases. Am J Surg Pathol. 2015;39:251–9.
Sung MT, Jones TD, Beck SD, Foster RS, Cheng L. OCT4 is superior to CD30 in the diagnosis of metastatic embryonal carcinomas after chemotherapy. Hum Pathol. 2006;37:662–7.
O’Shaughnessy MJ, Feldman DR, Carver BS, Sheinfeld J. Late relapse of testicular germ cell tumors. Urol Clin North Am. 2015;42:359–68.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gallo, A., Fankhauser, C., Hermanns, T. et al. HNF1β is a sensitive and specific novel marker for yolk sac tumor: a tissue microarray analysis of 601 testicular germ cell tumors. Mod Pathol 33, 2354–2360 (2020). https://doi.org/10.1038/s41379-020-0597-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-020-0597-x
This article is cited by
-
SMARCB1 (INI1)-Deficient Sinonasal Carcinoma with Yolk Sac differentiation Showing Co-loss of SMARCA4 Immunostaining – A Case Report and Literature Review
Head and Neck Pathology (2022)
-
HNF-1β as an immunohistochemical marker for distinguishing chromophobe renal cell carcinoma and hybrid oncocytic tumors from renal oncocytoma
Virchows Archiv (2021)