Abstract
Dedifferentiated liposarcoma represents a form of liposarcoma composed of a non-lipogenic sarcoma associated with well-differentiated liposarcoma. The prognostic significance of histological grading of the dedifferentiated component remains to be elucidated due to vague grading criteria employed in previous studies. Molecular markers of tumor behavior, including amplification levels of murine double minute-2 (MDM2) and cyclin-dependent kinase-4 (CDK4) genes, have been explored in a limited number of cases. Here we investigate whether ‘Fédération Nationale des Centres de Lutte Contre le Cancer’ (FNCLCC) grade and MDM2 gene amplification levels have prognostic value in dedifferentiated liposarcoma in terms of local recurrence and disease-specific survival. Fifty cases were retrieved, reviewed and FNCLCC grade was scored for the dedifferentiated component. Testing for MDM2 gene amplification was performed by fluorescence in situ hybridization. Amplification was categorized as high level (≥20 copies) and as low level (<20 copies). Follow-up data was obtained through chart review. Log-rank test and Cox proportional hazard models were used to determine the effect of grade and level of MDM2 amplification on outcomes. Our series includes 50 patients (male n=28, female n=22) with an average age of 63 years (range, 28–88) and a median follow-up of 28 months (range, 2–120). Tumors were graded as grade 1 (6%), grade 2 (58%), and grade 3 (36%). When adjusted for age, sex, site, tumor size, and margin status, grade 3 patients had a higher recurrence rate than grades 1 and 2 (HR=2.07, 95% CI: 1.24, 7.62; P=0.015). Patients with high-level MDM2 amplification had higher recurrence rate on univariate analysis (P=0.028), but not on multivariate analysis (HR=1.69, 95% CI: 0.73, 3.94; P=0.221). FNCLCC grade 3 dedifferentiation confers a worse prognosis in dedifferentiated liposarcoma in terms of local recurrence. MDM2 amplification level remains a useful diagnostic tool in dedifferentiated liposarcoma, but has no prognostic value in terms of local recurrence.
Similar content being viewed by others
Main
Liposarcoma is the most common soft-tissue sarcoma in adults. The World Health Organization divides liposarcomas into four subtypes: atypical lipomatous tumor/well-differentiated liposarcoma, myxoid liposarcoma, dedifferentiated liposarcoma, and pleomorphic liposarcoma.1 Dedifferentiated liposarcoma consists of atypical lipomatous tumor/well-differentiated liposarcoma associated with a non-lipogenic dedifferentiated component in the primary tumor or in a recurrence. Dedifferentiation is believed to confer aggressive behavior at least with respect to the potential for metastatic disease.2 However, only a limited number of studies have methodically investigated pathological and clinical parameters that may predict outcome. In a study by Henricks et al,3 retroperitoneal tumors had a significantly worse outcome than those arising at other sites. The extent of dedifferentiation does not appear to have prognostic significance; however, a minimal amount of dedifferentiation that has biological significance has not been established.2, 3 The histological appearance or line of differentiation of the non-lipogenic component has also not been found to predict outcome.2, 3 Overall, apart from the anatomic site, there have been no reliable prognostic factors identified.
Historically, the significance of grading dedifferentiated liposarcoma has been a subject of debate. The general consensus has been that the non-lipogenic component can be low or high grade and that both low- and high-grade dedifferentiated liposarcomas have similar behavior.2, 3 However, some authors continue to emphasize that only high-grade dedifferentiation is biologically relevant and should be the defining criteria for dedifferentiated liposarcoma.4, 5 Although the similar behavior between low- and high-grade dedifferentiated liposarcomas could be due to sampling error, as high-grade dedifferentiated liposarcoma often also has low-grade appearing areas, criteria employed for grading dedifferentiated liposarcoma have been variable and vague, often consisting of general histological descriptions such as low-grade appearing fibromatosis-like and high-grade appearing similar to an undifferentiated pleomorphic sarcoma (malignant fibrous histiocytoma-like).3 The ‘Fédération Nationale des Centres de Lutte Contre le Cancer’ (FNCLCC) grading system has become a reliable and consistent method for grading spindle cell and pleomorphic sarcomas, but the prognostic value of grading the dedifferentiated component in dedifferentiated liposarcoma using the FNCLCC system is currently unknown.
Over the last two decades, our understanding of the molecular pathology of liposarcomas has evolved. It is now well known that atypical lipomatous tumor/well-differentiated liposarcoma and dedifferentiated liposarcoma are characterized by amplified sequences of chromosomal region 12q14-15, which contains the murine double minute-2 (MDM2) and cyclin-dependent kinase-4 (CDK4) genes. These molecular events are consistently identified in these tumors and represent the main driver event.6, 7, 8 To date, the role of these molecular abnormalities as predictive biomarkers for tumor behavior and prognosis has been explored in only a limited number of dedifferentiated liposarcoma cases.9 The purpose of our study was to determine whether there are biomarkers that can be combined with morphological parameters to begin developing a risk stratification system or molecular grading system for dedifferentiated liposarcoma. Specifically, we analyzed a large series of dedifferentiated liposarcoma to determine whether FNCLCC grade and MDM2 gene amplification levels have prognostic value in dedifferentiated liposarcoma in terms of local recurrence and disease-specific survival.
Materials and methods
Patient Selection
Institutional pathology database was reviewed to identify patients with dedifferentiated liposarcoma treated at our medical center between July 2000 and June 2013 inclusive. Seventy-two cases were selected. Of these, 20 cases were excluded as they represented local recurrences of previously treated dedifferentiated liposarcoma at outside institutions.
Pathologic Review
All cases were reviewed by a soft-tissue pathologist. The diagnosis was confirmed by morphological, immunohistochemical findings and previous cytogenetic or MDM2 gene amplification analysis available for review. Dedifferentiated liposarcoma was defined as a region devoid of lipogenic differentiation occupying at least 10% of the tumor or two continuous low-power ( × 4 objective) microscopic fields.9 Sclerosing well-differentiated liposarcoma cases with a large sclerosing component accounting for 10% of the tumor or occupying two continuous low-power fields were not included. All our cases showed discrete areas completely devoid of adipocytic cells with higher cellularity than that expected to be seen in sclerosing well-differentiated liposarcoma. MDM2 amplification studies were performed on 47 cases. In the remaining three cases, material was not available for molecular testing. Pathologic diagnostic criteria for dedifferentiated liposarcoma included the presence of a transition from an atypical lipomatous tumor/well-differentiated liposarcoma to a non-lipogenic sarcoma of variable histological grade. Pure high-grade spindle cell sarcomas lacking a characteristic well-differentiated component were excluded from the study to avoid including other histological types of sarcomas. The FNCLCC grading system was used in our study. Tumors were scored for tumor differentiation (score 1–3), mitotic count (score 1–3), and tumor necrosis (0–2) (Figure 1). Although applying the FNCLCC criteria, we emphasized the importance of tumor differentiation scoring, resulting in a ‘modified’ FNCLCC grading system. Dedifferentiated areas were assigned a differentiation score of 3 when they showed areas with large pleomorphic cells with severe atypia and no evidence of potential line of differentiation morphologically (that is, undifferentiated pleomorphic sarcoma-like morphology). Tumors with bland spindle cell morphology and mild atypia with morphology simulating benign or low-grade fibroblastic neoplasms (that is, fibromatosis-like) were given 1 as a differentiation score. Tumors with a moderate degree of atypia and/or certain line of differentiation morphologically were given 2 as a differentiation score (that is, myxofibrosarcoma-like; Figure 2). Grade was assigned based on the highest grade present in the dedifferentiated component rather than the most predominant grade (that is, a case with 80% of the dedifferentiated component grade 2 and 20% grade 3 was scored as a grade 3). Single scattered large atypical cells similar to those in atypical lipomatous tumor were not considered eligible for a differentiation score of 3 unless they were part of a well-formed focus of more diffuse atypia within the dedifferentiated component. Of note, we did not encounter any of these scattered cells in our cases.
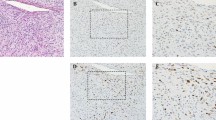
Hematoxylin–eosin stain (a–d). (a) × 10, dedifferentiated liposarcoma with a well-differentiated liposarcoma/atypical lipomatous tumor component adjacent to a spindle cell non-adipocytic sarcomatous component. (b) × 20, ‘modified’ Fédération Nationale des Centres de Lutte Contre le Cancer (FNCLCC) grade 1 dedifferentiated component consisting of spindle cells showing bland cytological features with only mild atypia and no evidence of necrosis. (c) × 20, ‘modified’ FNCLCC grade 2 dedifferentiated component with cells showing a moderate degree of atypia (arrow) with increased mitotic activity (circle) and no necrosis. (d) × 20, ‘modified’ FNCLCC grade 3 dedifferentiated component with hypercellular areas showing severe nuclear atypia and pleomorphism with increased mitotic activity (circles) and coagulative necrosis (inset).
Hematoxylin–eosin stain (a–c). (a) × 20, a case with a tumor differentiation score of 1 showing bland spindle cell morphology (fibromatosis-like morphology) and mild atypia. (b) × 20, a tumor with moderate nuclear atypia and pleomorphism (myxofibrosarcoma-like morphology) that was assigned a differentiation score of 2. (c) × 20, tumors with areas showing large pleomorphic cells with severe atypia and bizarre hyperchromatic nuclei (pleomorphic undifferentiated sarcoma-like morphology) were assigned a differentiation score of 3.
Immunohistochemistry
In 15 selected cases, immunohistochemical studies were performed to further classify the dedifferentiated component. On-board epitope retrieval was accomplished according to the manufacturers’ instructions. Immunostaining for smooth muscle actin, desmin, S100, CD34, and MDM2 was accomplished with a fully automated, random access, open-system immunostainer (Bond IIITM—Leica Microsystems) using a polymer approach. The immunohistochemistry with primary antibodies directed against smooth muscle actin (monoclonal 1A4 1:200, Cellmarque, CA), desmin (monoclonal D33, 1:800, Dako, Denmark), S100 (monoclonal DR96+BC96, 1:1600, Biocare medical, CA), CD34(monoclonal qbend, 1:200, Dako, Denmark), and MDM2 (monoclonal IF2, 1:50, Calbiochem, MA) was performed. The chromogen diaminobenzidine tetrachloride was used to visualize the antibody–antigen complex. The tissue was counterstained with hematoxylin. Appropriate positive and negative control slides were prepared. The negative controls consisted of slides processed without addition of the primary antigen.
Fluorescence In Situ Hybridization Studies and Analysis
Fluorescence in situ hybridization (FISH) was performed in 47 cases according to the validated laboratory protocol. Briefly, unstained tissue sections were prepared and regions of the neoplasm for analysis were marked by the pathologist in comparison with H&E-stained sections from the same block. FISH for the MDM2 gene was then carried out using the FDA-approved SpectrumOrange Vysis LSI (Abbott), MDM2 FISH probe. The slides were counterstained in phosphate-buffered saline containing 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI II), p-phenylenediamine, and glycerol (Abbott), and then examined under a fluorescent microscope (Olympus, Tokyo, Japan) with Filter set (Abbott) for detecting DAPI II, Spectrum Orange, Spectrum Green and Spectrum Red. Cases were scored by counting a minimum of 40 nuclei per case under oil immersion at × 100 magnification.
Using DAPI filter, overlapping tumor nuclei were also excluded from the count to decrease false-positive scoring. At least two bright fluorescent spots per nucleus in 80% of cells were needed to consider the test interpretable. An average number of MDM2 signals was then calculated for each case. Amplification was defined as greater than five fluorescent signals per cell. In our study, low amplification level was defined as an average of <20 fluorescent signals per case. High amplification level was defined as ≥20 fluorescent signals per case (Figure 3). We chose 20 as a cutoff as this number represented a median value around which the cases showed a near normal distribution.
Fluorescence in situ hybridization and immunohistochemical studies for murine double minute-2 (MDM2) amplification levels and MDM2 protein expression (a–c). (a) Low-level MDM2 amplification evidenced by more than five MDM2 copies per nucleus; the average MDM2 orange signals’ count per nucleus was 18 in this case (<20). (b) High-level MDM2 amplification evidenced by many signals per nucleus; the average MDM2 orange signals’ count per nucleus was 30 in this case (>20). (c) MDM2 immunostain showing diffuse intense nuclear expression correlating with MDM2 amplification in all cases (n=15) where the immunostain was performed for diagnostic purposes.
Follow-up Information and Statistical Analysis
Clinical follow-up information was obtained from hospital electronic records and telephone calls to treating clinician in 49 cases. One patient was lost to follow-up and FISH analysis could not be performed on the tissue from the tumor. This patient was excluded from further statistical analysis. The Student two-sided t-test was used to detect differences for continuous variables. Pearson χ2 test was used to determine correlations between prognostic variables. Survival curves were constructed using the methods of Kaplan and Meier with local recurrence-free survival and disease-specific survival as end points. Time to these events was calculated beginning with the date of initial surgery. Statistical analysis for survival curves was based on the log-rank test. Cox proportional hazard models was used for multivariate analysis.
Results
Clinical and Pathological Findings
For the 13-year period under study, we identified 50 cases of dedifferentiated liposarcoma treated at our institution having sufficient pathologic material at the time of this study for further evaluation. Clinicopathological data related to the patients and tumors in the study group are shown in Table 1. Tumor size ranged from 3.5 to 55 cm and the mean tumor size was 20.7 cm. Tumors were located in the retroperitoneum (68%), the extremities (18%), and other sites (14%). By FNCLCC criteria, 36% of tumors were high grade, 58% intermediate grade, and 6% low grade. Among the 50 cases, 44 had microscopically positive margins defined as tumor present at or within 0.1 cm from the inked margin. Of these, six cases showed well-differentiated tumor at the margin, whereas the remaining 38 tumors had the dedifferentiated component present at the margin. Fifteen cases showed features consistent with myofibroblastic differentiation with at least focal-positive immunoreactivity with smooth muscle actin (n=15) and/or desmin (n=6). CD34 showed focal-to-variable positive immunoreactivity in five of the cases that was labeled with smooth muscle actin. No positive immunoreactivity was detected with S100 in any of the tested cases. Also, three cases exhibited heterologous metaplastic bone formation. In all other cases, no definitive line of differentiation could be identified. When available, MDM2 immunostain showed diffuse nuclear expression in all 15 tested cases, which correlated with amplification of the MDM2 gene by FISH studies (Figure 3). Cytogenetic studies were available in 18 cases. Six cases showed normal cell growth with suboptimal results for interpretation. Two cases showed normal karyotype and two other cases showed complex karyotype, whereas the eight remaining cases showed giant ring chromosome typically associated with dedifferentiated liposarcoma.
Treatment and Follow-up
All of our patients underwent surgical resection with curative intent. Within this group of patients, 29 patients were treated with surgical resection only with planned close follow-up for a period of 5 years. Within this subgroup, the tumor was located in the retroperitoneum in 21 cases. Five cases were treated with neoadjuvant chemotherapy (n=2), neoadjuvant radiation (n=2), or a combination of both (n=1) with subsequent surgical resection. Fourteen patients received adjuvant therapy with surgical resection including radiation (n=10) and radiation with chemotherapy (n=4). Median follow-up for the patients treated surgically at our institution was 28.3 months (range, 1–121 months). Twenty patients developed local recurrence and/or distant metastasis that occurred from 9 to 100 months of follow-up. Within this group, three patients with local recurrences also developed distant metastases (diagnosed at the same time as the local recurrence). Also, two patients developed metastases after surgical resection (12–22 months after surgery). Five patients died of their disease (5/50; 1–13 months after surgery). Twenty-four patients showed no evidence of disease at the time of last follow-up (range, 2–120 months).
MDM2 Gene Amplification
All of the 49 cases analyzed showed greater than five fluorescent signals per nucleus. In our series, low amplification level was seen in 57% of cases with an average of <20 fluorescent signals per nucleus. High amplification level was seen in 43% of cases with an average of >20 fluorescent signals per nucleus (range, 6.6–34.6 fluorescent signals per nucleus; Table 1).
Correlation of Different Clinicopathological Parameters
To determine the clinical significance of MDM2 gene amplification level, we correlated this parameter with other clinicopathological parameters. Analysis of the entire cohort demonstrated no significant association (P>0.05) between MDM2 amplification levels (defined as either high or low level of amplification) and FNCLCC grade, margin status, size, site, age, and sex (Table 2). Furthermore, there was no significant statistical difference in the MDM2 amplification levels across different grade groups (P=0.56). These are interesting findings as they demonstrate that previously reported relevant pathological parameters such as site and margin status are independent of MDM2 level of amplification (Table 3).
Analysis of Clinicopathological Parameters for Local Recurrence/Distant Metastasis and Disease-Specific Survival
We analyzed the significance of MDM2 amplification levels and FNCLCC grade of the dedifferentiated component with local recurrence-free survival and disease-specific survival of our patients (n=49). As illustrated in Figure 4, univariate analysis showed that patients with grade 3 tumors have a worse local recurrence-free survival compared with patients with grade 1 and 2 tumors (P=0.03). Similarly, high amplification levels (>20 gene copies) were associated with worse local recurrence-free survival compared with low amplification levels (<20 gene copies; P=0.02) on univariate analysis (Figure 4).
Univariate analysis of local recurrence-free survival of 49 patients treated surgically at a single institution for dedifferentiated liposarcoma. Dedifferentiated liposarcomas with a grade 3 dedifferentiated component have decreased local recurrence-free survival compared with grades 1 and 2, P=0.03 (upper right). Dedifferentiated liposarcoma patients with high murine double minute-2 (MDM2) amplification levels have decreased local recurrence-free survival compared with patients with low MDM2 amplification levels, P=0.02 (lower right). Dedifferentiated liposarcoma patients treated with surgery alone show a statistically nonsignificant trend toward worse local recurrence-free survival compared with patients treated with surgery and a combination of radiation and chemotherapy (P=0.22; upper left). Dedifferentiated liposarcoma patients with negative microscopic margins show a statistically nonsignificant trend toward a better local recurrence-free survival compared with patients with positive microscopic margins (P=0.32; lower left). The Kaplan–Meier method was used to estimate local recurrence probability and the log-rank method was used to compare the curves. The Kaplan–Meier curves are not shown past 5 years because no patients were observed beyond that point. Amp, amplification; LR, local recurrence.
Multivariate analysis was performed to further explore the importance of FNCLCC grade and MDM2 amplification levels with regard to other clinicopathological variables such as size, tumor site, age, sex, margin status, and treatment modality. High tumor grade (grade 3) conferred a worse prognosis in terms of local recurrence compared with non-high tumor grade (grades 1 and 2), HR=3.07 (95% CI: 1.24, 7.62; P=0.015). However, amplification level (high vs low) did not reach statistical significance as an independent predictive factor associated with local recurrence on multivariate analysis, HR=1.92 (95% CI: 0.81, 4.58; P=0.138). The overall disease-specific survival analysis was not possible due to limited number of disease-related deaths (n=5). This resulted in inadequate statistical power to compare DFS time differences between groups.
In addition, we sought to determine the effect of treatment modalities on local recurrence. For this purpose, we analyzed the correlation between treatment (surgery vs surgery with combination chemotherapy and/or radiation therapy) and local recurrence in our patients. There was no significant statistical difference in terms of local recurrence between the two groups (P=0.22). Although multivariate analysis showed that the hazard of recurrence in the combinational treatment group may be smaller than the surgery-only group, HR=0.73, such a difference was not statistically significant (P=0.480; Table 4).
Discussion
As Evans introduced the term ‘tumors with distinct areas of well-differentiated liposarcoma and cellular non-lipogenic spindle cell or pleomorphic sarcoma,’4 dedifferentiated liposarcoma has become a well-known entity characterized by amplified chromosomal region 12q13-15, which contains the MDM2 and CDK4 genes, among others. MDM2 is an oncogene that promotes degradation of tumor suppressor protein p53 and was shown to be consistently amplified in dedifferentiated liposarcoma in association with a HMGA2 as a gene partner.10 CDK4 is located at 12q14.1 and is frequently amplified in tumors that have amplified MDM2. CDK4 phosphorylates retinoblastoma (RB) 1; this phosphorylation disrupts RB’s interaction with E2F transcription factors and allows the cell cycle to proceed through the G1-S checkpoint.11
Recently, in a series of 177 spindled and pleomorphic tumors, Kimura et al12 reported 100% sensitivity and 95% specificity for MDM2 amplification in distinguishing dedifferentiated liposarcoma from spindle and pleomorphic sarcomas. The authors also demonstrated that MDM2 overexpression has 100% sensitivity and 87% specificity in distinguishing dedifferentiated liposarcoma from other tumors. In their study, Weaver et al showed 100% sensitivity for MDM2 in distinguishing between benign lipomatous tumors and WDLS/dedifferentiated liposarcoma. The authors also showed that the specificity of this test decreases in high-grade sarcomas essentially when trying to distinguish between dedifferentiated liposarcoma and pleomorphic sarcomas; 40% of a limited number of pleomorphic sarcomas tested showed MDM2 amplification.13 Using immunohistochemical methods, Binh et al14 reported a sensitivity of 97% and a specificity of 92% for MDM2 expression in distinguishing well-differentiated liposarcomas/atypical lipomatous tumors and dedifferentiated liposarcomas from other soft-tissue neoplasms. In line with these published results, all our tested dedifferentiated liposarcoma cases showed MDM2 amplification, except for one case that failed interpretation, yielding a sensitivity of ∼99.8% for the FISH-testing method. In addition, all cases that showed MDM2 overexpression had MDM2 amplification. This finding supports the previously reported results (MDM2 overexpression had a 100% sensitivity) by Kimura et al12 and Binh et al.14
Dedifferentiated liposarcoma represents a progression of well-differentiated liposarcoma, which has no metastatic potential, into a sarcoma with potential for metastatic disease and possibly more locally aggressive behavior. The full scope of the clinical significance of dedifferentiation and the molecular events leading to such progression is poorly understood. Recently, specific chromosomal copy number abnormalities associated with dedifferentiation were identified in dedifferentiated liposarcoma, but not in well-differentiated liposarcoma. Namely, 11q23-24 region, which carries several genes that are downregulated during dedifferentiation, was lost in 79% of the dedifferentiated liposarcoma-tested cases.15 To date, there are few studies investigating molecular prognostic markers in dedifferentiated liposarcoma. In a series of 143 cases of well-differentiated liposarcoma/dedifferentiated liposarcoma, Italiano et al16 demonstrated that well-differentiated liposarcoma/dedifferentiated liposarcoma with MDM2 amplification but no CDK4 amplification has a favorable prognosis with lower rate of recurrence. Recently, Lee et al9 investigated the prognostic significance of MDM2 and CDK4 amplification levels in a series of 56 well-differentiated liposarcoma/dedifferentiated liposarcoma using both FISH and quantitative PCR and identified high amplification levels of CDK4 as an independent adverse prognostic factor affecting overall survival and disease progression. MDM2 levels of amplification did not show any prognostic significance. Our results are in line with these findings demonstrating no significant prognostic effect of MDM2 amplification level. Yet, two important facts related to the study design are worth being noted. The method and cutoff used in the previous series are different from ours. We based our stratification of high and low amplification levels on FISH signal detection, whereas the other series used quantitative PCR. Also, we identified 20 signals per nucleus as a cutoff between the high and low levels, whereas the previous series used 10 copy numbers as a cutoff for high level of amplification. Taking our results and the previously published data by Lee et al, one can conclude that MDM2 amplification remains a useful diagnostic marker for dedifferentiated liposarcoma, but does not have a role in risk stratification of patients with dedifferentiated liposarcoma. However, CDK4 amplification level appears to be a promising prognostic biomarker in dedifferentiated liposarcoma. The reason for the difference in the clinical impact of amplification of both of these oncongenes (MDM2 and CDK4) located within the same amplified region (12q13-15) remains unclear.
Grading of soft-tissue sarcomas is problematic as it historically has relied on generic, vague, and subjective histological criteria. More specifically, previous studies attempting to define and understand the clinicopathological significance of dedifferentiated liposarcoma have not used standard grading criteria, but rather have used more descriptive definitions of low- and high-grade dedifferentiated liposarcoma.2, 3, 9 The FNCLCC grading system has shown value in predicting distant metastasis and overall survival, but not local recurrences, in the main histological subtypes of sarcomas,17 and currently is accepted as the preferred method for grading sarcomas (when grading is appropriate for a given subtype). Notably, there are no studies addressing the value of the FNCLCC grading system in predicting outcome, including local recurrence, in dedifferentiated liposarcoma. Weiss et al investigated potential clinicopathological prognostic markers in dedifferentiated liposarcoma and found that location was the only significant prognostic variable for survival, with retroperitoneal site carrying the worst prognosis. In their multivariate analysis, neither grade nor extent of dedifferentiation was found to be independent prognostic factors in dedifferentiated liposarcoma.2, 3 Importantly, the criteria for grading were not well defined and relied solely on the general subjective histological appearance of the tumor. Less-subjective criteria of mitotic activity and amount of necrosis utilized in the FNCLCC system were not included. In a study of 177 retroperitoneal liposarcomas, Singer et al18 showed that dedifferentiated liposarcoma was associated with a fourfold increased risk of local recurrence compared with well-differentiated liposarcoma. Similar results were reported by the same group in 2006; the authors showed worse 5-year disease-specific survival rates in dedifferentiated liposarcoma (44%) compared with well-differentiated liposarcoma (93%).19 Neither series examined the effect of grade of dedifferentiation in the dedifferentiated liposarcoma subgroup on local recurrence and disease-specific survival. Recently, Lee et al identified histological grade as an independent prognostic factor predicting worse disease-specific survival in a series of 56 liposarcoma including 26 dedifferentiated liposarcoma. The authors demonstrated that patients with dedifferentiated liposarcoma high grade had a significantly worse disease-specific survival compared with patients with well-differentiated liposarcoma/dedifferentiated liposarcoma low grade.9 Nevertheless, their multivariate analysis did not identify grade as an independent prognostic factor for local recurrence. These authors also did not utilize FNCLCC grading criteria when grading dedifferentiated liposarcoma cases. Our results clearly identify FNCLCC grade as an independent prognostic factor predicting local recurrence in dedifferentiated liposarcoma. Unfortunately, conclusions concerning disease-specific survival could not be drawn in our group due to the limited number of disease-related deaths (n=5).
One can argue that in our study, scoring for differentiation does not adhere to the ‘standard’ FNCLCC grading system that assigns a differentiation score of 3 to all dedifferentiated liposarcoma. In fact, the rationale behind using this ‘modified’ FNCLCC grading system (tumors assigned a differentiation score 1–3) for the dedifferentiated component similar to other adult sarcomas was based on the hypothesis that dedifferentiated component behaves differently based on degree of differentiation and should be scored as such. Furthermore, this allowed for inclusion of a low-grade dedifferentiated liposarcoma category, a category previously vaguely defined, but thought to have similar behavior to high-grade dedifferentiated liposarcoma. In fact, performing multivariate analysis using the ‘standard’ and ‘modified’ FNCLCC showed similar results; HR=3.07 (95% CI: 1.24, 7.62; P=0.015) and HR=3.25 (95% CI: 1.55, 8.82; P=0.018), respectively. In both grading schemes, it was clear that high-grade dedifferentiated liposarcoma was three times more likely to recur compared with non-high-grade dedifferentiated liposarcoma (grades 1 and 2 tumors). Furthermore, similar results were obtained using the ‘standard’ FNCLCC system when performing correlation analysis (results not shown in tables).
Our findings indicate that subclassification of dedifferentiated liposarcoma by histological grade of the dedifferentiated component according the FNCLCC system predicts local recurrence. Furthermore, these findings reinforce the belief that dedifferentiated liposarcoma is indeed a heterogeneous group of tumors with different behaviors. More specifically, the biology of low-grade dedifferentiated liposarcoma (FNCLCC grades 1 and 2) may be distinct from that of high-grade dedifferentiated liposarcoma (FNCLCC grade 3). This contrasts with previous reports showing no association between histological grade in dedifferentiated liposarcoma and clinical outcome.3 One can argue that grading of the dedifferentiated component in dedifferentiated liposarcoma is a difficult task that is prone to a significant amount of subjectivity regardless of the grading system utilized. Yet, in both series (ours and the series published by Lee et al), a dichotomous system was used to compare tumor grades. FNCLCC grade 3 tumors were compared with FNCLCC grades 1 and 2 tumors in our series. Dedifferentiated liposarcoma high grade was compared with dedifferentiated liposarcoma low grade/well-differentiated liposarcoma in the series by Lee et al.9 This dichotomous stratification seems to allow for more meaningful risk assessment of patients at least in terms of local recurrence and possibly for disease-specific survival.
Margin status has been shown to be one of the most important predictors of local recurrences in sarcomas. There are three potential causes of local recurrence. First, intracapsular resection of soft-tissue sarcoma in which gross residual tumor is left behind will inevitably lead to local recurrence. Second, microscopically ‘negative’ margins incur varying risk of leaving focally infiltrative margins or satellite nodules, which have been found up to 4 cm beyond the pseudocapsule. Third, despite truly complete primary resection, there is a theoretical risk that circulating tumor cells may re-seed the wound bed and cause a local recurrence. Surgically traumatized tissue has a favorable milieu for malignant cell implantation.20, 21 In our series, multivariate analysis did not identify margin status as an independent predictive prognostic factor for local recurrence in dedifferentiated liposarcoma. Although different from the previously reported data by Lee et al,9 this finding is not surprising and can be explained by two facts. First, the series reported by Lee et al included both well-differentiated liposarcoma and dedifferentiated liposarcoma, whereas ours focused on dedifferentiated liposarcoma exclusively. Dedifferentiated liposarcoma occurs much more often in the retroperitoneum than well-differentiated liposarcoma, which may confer a higher recurrence rate when compared with well-differentiated liposarcoma. This may weigh in the statistical analysis against the margin status as an independent prognostic factor. Second, 88% of our dedifferentiated liposarcoma cases had a positive margin and 70% of them were retroperitoneal. This fact may have had a ‘randomization’ effect in our series that highlighted the independent effect of tumor grade on local recurrence. Further studies with larger patient numbers are needed to validate our findings.
In sum, we present solid evidence that, despite its shortcomings, the FNCLCC grading system is a valuable tool for predicting local recurrence in dedifferentiated liposarcoma. Our results serve as a rationale to include FNCLCC grading of the dedifferentiated component of dedifferentiated liposarcoma in pathology reports, and provide a reason to consider more aggressive local control measures in the management of patients with FNCLCC grade 3 dedifferentiated liposarcoma. Although well established as a diagnostic tool, MDM2 amplification level is not a significant prognostic factor in dedifferentiated liposarcoma. Recently, CDK4 amplification has been identified as a promising prognostic factor in WDL and dedifferentiated liposarcoma.9 Additional analysis confirming the later findings and studies aimed at discovering additional prognostic molecular biomarkers in dedifferentiated liposarcoma, such as 11q23-24 deletion, are warranted to identify the most useful molecular markers to be used for prognostic purposes. These molecular markers can be combined with FNLCCC grade to develop a molecular grading (outcome risk stratification) system for dedifferentiated liposarcoma that will allow for more precise treatment and management of these patients.
References
Dei Tos J . Dedifferentiated liposarcoma In Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. (eds) WHO Classification of Tumors of Soft Tissue and Bone. International Agency for Research on Cancer (IRAC): Lyon, France, 2013, pp 37–38.
Weiss SW, Rao VK . Well differentiated-liposarcoma (atypical lipoma) of deep soft tissue of the extremities, retroperitoneum, and miscellaneous sites: a follow-up study of 92 cases with analysis of the incidence of ‘dedifferentiation’. Am J Surg Pathol 1992;16:1051–1058.
Henricks WH, Chu YC, Goldblum JR et al. Dedifferentiated liposarcoma: a clinicopathological analysis of 155 cases with a proposal for an expanded definition of dedifferentiation. Am J Surg Pathol 1997;21:271–281.
Evans HL . Liposarcoma: a study of 55 cases with a reassessment of its classification. Am J Surg Pathol 1979;3:507–523.
Evans HL . Atypical lipomatous tumor, its variants, and its combined forms: a study of 61 cases, with a minimum follow-up of 10 years. Am J Surg Pathol 2007;31:1–14.
Pilotti S, Della Torre G, Lavarino C et al. Distinct mdm2/p53 expression patterns in liposarcoma subgroups: implications for different pathogenetic mechanisms. J Pathol 1997;181:14–24.
Sirvent N, Forus A, Lescaut W et al. Characterization of centromere alterations in liposarcomas. Genes Chromosomes Cancer 2000;29:117–129.
Meis-Kindblom JM, Sjögren H, Kindblom LG et al. Cytogenetic and molecular genetic analyses of liposarcoma and its soft tissue simulators: recognition of new variants and differential diagnosis. Virchows Arch 2001;439:141–151.
Lee SE, Kim YJ, Kwon MJ et al. High level of CDK4 amplification is a poor prognostic factor in well-differentiated and dedifferentiated liposarcoma. Histol Histopathol 2014;29:127–138.
Italiano A, Bianchini L, Keslair F et al. HMGA2 is the partner of MDM2 in well differentiated and dedifferentiated liposarcomas whereas CDK4 belongs to a distinct inconsistent amplicon. Int J Cancer 2008;122:2233–2241.
Brown VD, Phillips RA, Gallie BL . Cumulative effect of phosphorylation of pRB on regulation of E2F activity. Mol Cell Biol 1999;19:3246–3256.
Kimura H, Dobashi Y, Nojima T et al. Utility of fluorescence in situ hybridization to detect MDM2 amplification in liposarcomas and their morphological mimics. Int J Clin Exp Pathol 2013;6:1306–1316.
Weaver J, Downs-Kelly E, Goldblum JR et al. Fluorescence in situ hybridization as a valid MDM2gene amplification as a diagnostic tool in lipomatous neoplasms. Mod Pathol 2008;21:943–949.
Binh MB, Sastre-Garau X, Guillou L et al. MDM2 and CDK4 immunostainings are useful adjuncts in diagnosing well-differentiated and dedifferentiated liposarcoma subtypes: a comparative analysis of 559 soft tissue neoplasms with genetic data. Am J Surg Pathol 2005;29:1340–1347.
Crago AM, Socci ND, DeCarolis P et al. Copy number losses define subgroups of dedifferentiated liposarcoma with poor prognosis and genomic instability. Clin Cancer Res 2012;18:1334–1340.
Italiano A, Bianchini L, Gjernes E et al. Clinical and biological significance of CDK4 amplification in well-differentiated and dedifferentiated liposarcomas. Clin Cancer Res 2009;15:5696–5703.
Coindre JM, Terrier P, Guillou L et al. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer 2001;91:1914–1926.
Singer S, Antonescu CR, Riedel E et al. Histologic subtype and margin of resection predict pattern of recurrence and survival for retroperitoneal liposarcoma. Ann Surg 2003;238:358–370.
Dalal KM, Kattan MW, Antonescu CR et al. Subtype specific prognostic nomogram for patients with primary liposarcoma of the retroperitoneum, extremity, or trunk. Ann Surg 2006;244:381–391.
White LM, Wunder JS, Bell RS et al. Histologic assessment of peritumoral edema in soft tissue sarcoma. Int J Radiat Oncol Biol Phys 2005;61:1439–1445.
Demicheli R, Retsky MW, Hrushensky WJM et al. The effects of surgery on tumor growth: a century of investigations. Ann Oncol 2008;19:1821–1828.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Jour, G., Gullet, A., Liu, M. et al. Prognostic relevance of Fédération Nationale des Centres de Lutte Contre le Cancer grade and MDM2 amplification levels in dedifferentiated liposarcoma: a study of 50 cases. Mod Pathol 28, 37–47 (2015). https://doi.org/10.1038/modpathol.2014.88
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2014.88
This article is cited by
-
Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Soft Tissue Tumors
Head and Neck Pathology (2022)
-
Update on genomic and molecular landscapes of well-differentiated liposarcoma and dedifferentiated liposarcoma
Molecular Biology Reports (2021)
-
Amplification of DNA damage-inducible transcript 3 (DDIT3) is associated with myxoid liposarcoma-like morphology and homologous lipoblastic differentiation in dedifferentiated liposarcoma
Modern Pathology (2019)
-
Activation of the Akt-mTOR and MAPK pathways in dedifferentiated liposarcomas
Tumor Biology (2016)
-
Up-regulation of annexin A2 expression predicates advanced clinicopathological features and poor prognosis in hepatocellular carcinoma
Tumor Biology (2015)