Abstract
Myxoid and round-cell liposarcoma is a frequently encountered liposarcoma subtype. The mainstay of treatment remains surgical excision with or without chemoradiation. However, treatment options are limited in the setting of metastatic disease. Cancer-testis antigens are immunogenic antigens with the expression largely restricted to testicular germ cells and various malignancies, making them attractive targets for cancer immunotherapy. Gene expression studies have reported the expression of various cancer-testis antigens in liposarcoma, with mRNA expression of CTAG1B, CTAG2, MAGEA9, and PRAME described specifically in myxoid and round-cell liposarcoma. Herein, we further explore the expression of the cancer-testis antigens MAGEA1, ACRBP, PRAME, and SSX2 in myxoid and round-cell liposarcoma by immunohistochemistry in addition to determining mRNA levels of CTAG2 (LAGE-1), PRAME, and MAGEA3 by quantitative real-time PCR. Samples in formalin-fixed paraffin-embedded blocks (n=37) and frozen tissue (n=8) were obtained for immunohistochemistry and quantitative real-time PCR, respectively. Full sections were stained with antibodies to MAGEA1, ACRBP, PRAME, and SSX2 and staining was assessed for intensity (1–2+) and percent tumor positivity. The gene expression levels of CTAG2, PRAME, and MAGEA3 were measured by quantitative real-time PCR. In total, 37/37 (100%) of the samples showed predominantly strong, homogenous immunoreactivity for PRAME. There was a variable, focal expression of MAGEA1 (11%) and SSX2 (16%) and no expression of ACRBP. Quantitative real-time PCR demonstrated PRAME and CTAG2 transcripts in all eight samples: six tumors with high mRNA levels; two tumors with low mRNA levels. The gene expression of MAGEA3 was not detected in the majority of cases. In conclusion, myxoid and round-cell liposarcomas consistently express PRAME by immunohistochemistry as well as CTAG2 and PRAME by qualitative real-time PCR. This supports the use of cancer-testis antigen-targeted immunotherapy in the treatment of this malignancy.
Similar content being viewed by others
Main
Myxoid and round-cell liposarcomas constitute approximately one-third of all liposarcomas, a relatively common group of fat-derived soft-tissue sarcomas.1 Surgery with or without chemoradiation therapy remains the mainstay of treatment. Unfortunately, treatment options in the setting of tumor recurrence, progression, and metastasis remain a challenge.2 Cancer-testis antigens are a unique family of antigens that have largely restricted expression toward testicular germ cells in a normal adult, yet are aberrantly expressed by a variety of histologically unrelated malignancies.3, 4 Furthermore, cancer-testis antigens are innately immunogenic, thus elicit spontaneous T-cell and/or humoral responses that target the cancer-testis antigen-expressing tumor cells.5, 6, 7
Cancer-testis antigen expression has been reported in a wide variety of malignancies, including melanoma, primary brain tumors, a variety of sarcomas and carcinomas, as well as hematologic neoplasms.7 Indeed, a few cancer-testis antigens, including MAGEA1, MAGEA3, and NY-ESO-1, have been targeted by immunotherapies in clinical trials.6 In addition to being therapeutic targets, cancer-testis antigen expression in certain tumors may provide prognostic value.8, 9, 10, 11, 12, 13, 14, 15 In most tumors, cancer-testis antigen expression is associated with worse outcome, higher tumor grade, and metastasis, however, there are exceptions.6, 16, 17 Given the potential utility of cancer-testis antigens in cancer therapeutics and prognostication, an extensive online database containing >100 cancer-testis genes has been established by the Ludwig Institute for Cancer Research (http://www.cta.lncc.br/).7
Similar to other sarcomas, gene expression studies have reported the presence of multiple cancer-testis antigens in liposarcomas, including SSX, CTAG2 (encodes LAGE-1), CTAG1B (encodes NY-ESO-1), CT-7, CT-10, GAGE, BAGE, PRAME, and various MAGE transcripts.18, 19, 20 Furthermore, increased CTAG1B, CTAG2, PRAME, and MAGEA9 mRNAs have been reported specifically in the myxoid and round-cell subtype.20, 21 More recently, overexpression of the highly immunogenic cancer-testis antigen NY-ESO-1 was reported in myxoid and round cell liposarcomas by both immunohistochemistry and quantitative real-time PCR.22, 23 Expression was seen in 90–100% of samples tested and immunoreactivity was strong and homogenous in the majority of positive cases. Of note, occasional expression was also reported in the pleomorphic and dedifferentiated liposarcoma subtypes.
Cancer-testis antigen-expressing tumors frequently demonstrate a coordinated expression of cancer-testis antigens, meaning more than one cancer-testis antigen is expressed.6 Given the consistent overexpression of NY-ESO-1 in myxoid and round cell liposarcoma, we evaluated for the expression of the cancer-testis antigens MAGEA1, ACRBP, PRAME, and SSX2 by immunohistochemistry and PRAME, CTAG2, and MAGEA3 by quantitative real-time PCR.
Materials and methods
Rationale
Frequent and homogenous expression of NY-ESO-1, a highly immunogenic cancer-testis antigen, in myxoid and round cell liposarcoma has been recently documented.20, 22, 23
Herein, we sought to explore the expression of other cancer-testis antigens as a rationale for a potential polyvalent immunotherapeutic target in the treatment of this neoplasm.
The MAGE antigens including MAGE1 and MAGE3 are attractive targets previously explored in immune-based clinical trials in solid organ malignancies. SSX2 and ACRBP are highly immunogenic antigenic targets and so are PRAME and CTAG2. There are immunotherapy-based clinical trials targeting PRAME, CTAG2 (in combination with CTAG1B) and SSX2 expression in various hematologic and solid organ malignancies.
Case Material
Myxoid and round cell liposarcomas (n=37) from 1992 to 2010 were retrospectively identified from the surgical pathology archives at Wexner Medical Center at The Ohio State University. The cases were re-reviewed by a bone and soft-tissue pathologist (OHI), and the diagnoses were confirmed by histomorphology per established morphologic criteria.24 Of the 37 cases of myxoid and round cell liposarcoma used in the study, 13 cases had been confirmed by a positive DDIT3 gene rearrangement determined by fluorescence in situ hybridization and/or karyotype analysis demonstrating a t(12;16)(q13;p11) translocation. In addition, frozen tissue of myxoid and round cell liposarcomas (n=8), well-differentiated liposarcomas (n=3) and dedifferentiated liposarcomas (n=3) was obtained for quantitative real-time PCR.
RNA Extraction and Quantitative Real-Time PCR
The mRNA expression of CTAG2 (encodes LAGE-1), PRAME (encodes PRAME), and MAGEA3 (encodes MAGE-A3) was measured by qualitative real-time PCR. Of note, the mRNA expression of CTAG1B (encodes NY-ESO-1) in these samples was reported previously.22 RNA was extracted from frozen sarcoma samples using Ribozol (Amresco, Solon, OH, USA) and a modified manufacturer’s protocol for RNA extraction using Trizol reagent (Ambion Life Technologies, Grand Island, NY, USA). RNA was quantitated using a NanoDrop-ND 1000 (Thermo Fisher Scientific, Wilmington, DE, USA). One microgram of RNA per sample was reverse transcribed using the iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA, USA). Qualitative real-time PCR was performed in 10 μl reactions according to recommended conditions by Applied Biosystems (Life Technologies). Taqman probes for GAPDH (Hs00266705_1), CTAG2 (Hs00535628_m1), PRAME (Hs01022301_m1), and MAGEA3 (Hs.PT.39a.22214836) were used. Input cDNA was doubled for the MAGEA3 due to low expression. Each sample was measured in triplicate. No template controls and no reverse transcriptase controls for each sample were included. Cycle threshold values were averaged across triplicate samples. GAPDH was used to calculate percentage relative expression of each sample. Samples were further normalized to the expression of the testis-positive control. Standard deviations were calculated by comparing delta cycle thresholds for each well in triplicate.
Immunohistochemistry
A representative formalin-fixed, paraffin-embedded block was obtained for each tumor (total n=37) and submitted for immunohistochemical staining with antibodies to ACRBP, MAGEA1, PRAME, and SSX2 (Table 1). Paraffin-embedded tissue sections (4 μm) on positively-charged slides were deparaffinized, rehydrated, and quenched for 5 min in a 3% hydrogen peroxide aqueous solution to block for endogenous peroxidase. Heat-Induced Epitope Retrieval (HIER) was performed in a vegetable steamer (Black & Decker, Madison, WI, USA) using 10X Target Retrieval Solution (pH 6.0; Dako, Carpinteria, CA, USA) for 25 min at 96 °C. Using the Dako Autostainer Immunostaining System, the sections were incubated with the primary antibody for one hour at room temperature. For MAGEA1, a detection system using biotinylated goat anti-mouse 2° antibody (Vector Laboratories, Burlingame, CA, USA) and Vectastain Elite 3° antibody (Vector Laboratories) was used. For ACRBP and PRAME, a detection system using biotinylated goat anti-rabbit 2° antibody (Vector Laboratories) and Vectastain Elite 3° antibody (Vector Laboratories) was used. The detection system used for the SSX2 monoclonal antibody was the MACH 3 Mouse HRP-Polymer Detection system (Biocare Medical, Concord, CA, USA). 3,3'-Diaminobenzidine (DAB; Dako) served as the chromogen for both the detection systems. The slides were counterstained in Richard Allen hematoxylin and evaluated. The Biotin Blocking System (Dako) was utilized with the ACRBP and PRAME antibodies.
Quantitation of Immunohistochemistry
Positive staining was scored semiquantitatively for the percent of tumor cells staining and intensity (0, negative; 1+, weak to moderate; 2+, strong), and subcellular location (cytoplasmic and/or nuclear).
Results
Quantitative Real-Time PCR
We measured CTAG2, PRAME, and MAGEA3 gene expression by quantitative real-time PCR in eight myxoid and round cell liposarcoma samples and one testis-positive control sample (Figures 1 and 2). Quantitative real-time PCR demonstrated CTAG2 and PRAME mRNA in all eight myxoid and round cell liposarcomas: six samples with high levels and two samples with low levels. As previously reported, seven (88%) samples showed gene expression of CTAG1B.22 Of note, the same two samples (#1 and #8) showed low expression levels of all three genes, consistent with the coordinated expression of CTAG1B, CTAG2, and PRAME. The MAGEA3 gene expression was detected in only two samples, both of which contained much lower levels compared with the testis control even when input cDNA was doubled. As several of the myxoid and round cell liposarcoma samples showed high levels of PRAME expression, we also assessed the level of PRAME in three well-differentiated liposarcomas and three dedifferentiated liposarcomas. One of the well-differentiated liposarcomas showed PRAME expression similar to the testis control. None of the dedifferentiated liposarcomas showed high levels of PRAME expression (Supplementary Figure 1).
Quantitative real-time PCR of myxoid and round cell liposarcomas samples. (a) CTAG2 (LAGE-1), (b) PRAME, (c) CTAG1B (NY-ESO-1) from previously published data,22 and (d) MAGEA3 expression in myxoid and round cell liposarcoma samples (1–8). Expression was normalized to GAPDH and is plotted at fold expression relative to testis (T).
Cancer-testis antigen expression by immunohistochemistry and quantitative real-time PCR in myxoid and round cell liposarcomas. The number of positive cases and the total number tested are enumerated above each data column. Of note, the MAGEA3 gene expression in both positive cases was below testis control levels. *Includes quantitative real-time PCR cases.
Immunohistochemistry
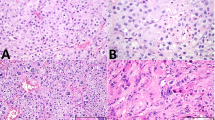
The results of cancer-testis antigen immunohistochemical staining in myxoid and round cell liposarcomas are summarized in Table 2 and Figures 2 and 3. In all, 37/37 (100%) of the cases showed immunoreactivity for PRAME seen primarily in a homogenous distribution (92% of positive cases showed >50% tumor cell positivity). Furthermore, the majority of positive cases (95%) demonstrated strong (2+) staining intensity for PRAME. MAGEA1 staining was only noted in four (11%) tumors, of which one case demonstrated a homogenous distribution. MAGEA1 staining intensity ranged from 1 to 2+. SSX2 immunoreactivity was noted in six (16%) tumors, seen in a focal distribution (2–25% of tumor cells). SSX2 staining intensity was primarily weak (1+) with only one case demonstrating 2+ staining. ACRBP was not expressed in any of the myxoid and round cell liposarcomas tested. For all positive instances of MAGEA1, PRAME, and SSX2, the staining was predominantly nuclear, and both the round cell and myxoid components stained similarly.
Discussion
Cancer-testis antigens are immunogenic antigens with an expression largely restricted to testicular germ cells and a variety of malignancies, making them attractive targets for cancer immunotherapy.5, 6 They can be classified into cancer-testis-X and non-X cancer-testis genes depending on genomic location on the X chromosome.25 The cancer-testis-X genes have been the principal targets of developing immunotherapies.6 The exact physiologic functions of most cancer-testis antigens are unknown; however, it is postulated that many are involved in germ cell self-renewal or differentiation, conferring tumorigenic qualities such as immortality, self-renewal, and migratory ability to cancer cells upon expression.6, 26
MAGEA1, MAGEA3, NY-ESO-1, LAGE-1, and SSX2 are all immunogenic cancer-testis-X antigens that have been shown to elicit coordinated humoral and cell-mediated immune responses.6, 7 Moreover, MAGEA1, MAGEA3, and NY-ESO-1 have been targeted in clinical trials investigating various immunotherapeutic approaches in melanoma, multiple myeloma, synovial sarcoma, and a variety of carcinomas, including lung and ovarian.6, 26, 27, 28, 29, 30, 31 Unfortunately, recently reported cross-reactivity between testis-restricted and non-testis-restricted MAGE antigens may ultimately limit clinical applications of highly active immunotherapies targeting the MAGE family antigens.32 The other two cancer-testis antigens evaluated in our study are both non-X cancer-testis antigens: PRAME, encoded on chromosome 22, and ACRBP, encoded on chromosomes 12.7 PRAME can elicit cytotoxic T cell-mediated immune responses as demonstrated in hematologic malignancies and melanoma and was recently targeted in a phase 1 clinical trial.33, 34, 35 ACRBP has been shown to elicit spontaneous humoral responses in a proportion of carcinomas, but has yet to be studied clinically.36, 37
Homogenous overexpression of the highly immunogenic cancer-testis antigen NY-ESO-1 was recently reported in the majority of myxoid and round cell liposarcomas, demonstrated by both quantitative real-time PCR and immunohistochemistry.22, 23 Commonly, expression of cancer-testis antigens within a tumor is coordinated; thus, more than one is expressed in a single tumor.6, 9 In the current study, we report an increased protein expression of PRAME, SSX2, and MAGEA1 in addition to the increased gene transcription of PRAME and CTAG2 (LAGE-1) in myxoid and round cell liposarcomas.
As determined by immunohistochemistry, PRAME was overexpressed in 100% of the myxoid and round cell liposarcomas evaluated in our study. The finding of PRAME overexpression is consistent with previous reports of an increased PRAME mRNA in myxoid and round cell liposarcomas.20, 21 Interestingly, we found very low levels of PRAME mRNA in dedifferentiated liposarcomas and very high level in one of the three well-differentiated liposarcomas, suggesting that PRAME may be a potential therapeutic target in well-differentiated liposarcomas. However, further investigation using a large sample cohort is warranted to evaluate the frequency of expression of PRAME in well-differentiated liposarcomas. In addition to expression in melanomas, sarcomas, and carcinomas, PRAME is unique among cancer-testis antigens; in that, it is frequently overexpressed in hematopoietic malignancies, including leukemia and lymphoma.7, 38, 39 Interestingly, PRAME appears to contribute toward tumorigenesis by inhibiting the retinoic acid receptor pathway, which prevents retinoic acid-induced proliferation arrest, differentiation, and apoptosis, ultimately conferring growth and survival advantages to cancer cells.40
LAGE-1, encoded by the CTAG2 gene, is a closely related homolog of NY-ESO-1, encoded by the CTAG1B gene.41 Both LAGE-1 and NY-ESO-1 are overexpressed in various cancer types, including melanoma, sarcoma, multiple myeloma, and a variety of carcinomas, including lung, head and neck, and ovarian.26, 41 In the current study, we described CTAG2 and PRAME transcripts in all eight myxoid and round cell liposarcoma samples, the majority of which demonstrated a higher expression than that seen in normal testis. Our data regarding PRAME and NY-ESO-1, reported here and previously, support correlated cancer-testis antigen mRNA and protein expression.22 In general, tumors with cancer-testis antigen mRNA levels of >10% testicular mRNA levels will demonstrate protein expression.6 Thus, it is likely that LAGE-1 protein is also frequently overexpressed in myxoid and round cell liposarcomas. Frequent expression of the homologs LAGE-1 and NY-ESO-1 enables the use of immunotherapies that target a common epitope to both antigens. For instance, McCormack et al (2012) recently reported promising preclinical data investigating the novel immunotherapeutic agent ImmTAC-NYE (Immune-mobilizing monoclonal T-cell receptors Against Cancer-NY-ESO-1).42 ImmTAC-NYE is a bi-specific fusion protein comprised of a monoclonal T-cell receptor specific for both NY-ESO-1 and LAGE-1 fused with anti-CD3, which activates a potent antitumor T-cell response.
We report an occasional MAGEA1 (11% of cases) and SSX2 (16% of cases) expression and essentially no expression of ACRBP and MAGEA3 in our samples of myxoid and round cell liposarcoma. Expression of MAGEA1, the first identified cancer-testis antigen, has been described in melanomas as well as various carcinomas and sarcomas, but has yet to be reported in myxoid and round cell liposarcomas.7, 18, 43 Interestingly, MAGE gene expression may protect tumor cells from apoptosis by interfering with p53 activity.44 SSX2 expression has been described in melanoma, multiple myeloma, and various carcinomas and sarcomas, including gene expression in liposarcomas.7, 18, 45 ACRBP mRNA has been detected in various cancers, including bladder, breast, lung, liver, and colon, and protein expression was reported in a majority of epithelial ovarian cancer.36, 37 SSX2, which appears to function as a transcriptional regulator, and ACRBP, which normally localizes to the sperm acrosome where it likely functions as a binding protein to proacrosin, are both listed as high priority targets for cancer therapy based upon criteria such as antigen specificity, oncogenicity, immunogenicity, expression level, and number of identified epitopes.36, 46, 47 MAGEA3, a member of the MAGE family, is one of the most frequently expressed cancer-testis antigens in cancer, and gene expression has previously been reported in liposarcomas, but not specifically in the myxoid and round cell subtype.7, 18, 48, 49 Unfortunately, the apparent lack of ACRBP and MAGEA3 expression in myxoid and round cell liposarcomas precludes their utility as an immunotherapeutic target in this particular neoplasm.
The overexpression of PRAME, CTAG2 (LAGE-1), and CTAG1B (NY-ESO-1) supports the use of cancer-testis antigen-targeted immunotherapy in the treatment of myxoid and round cell liposarcoma. Current immunotherapeutic strategies vary and include nonspecific immunomodulation, vaccines, and adoptive cellular therapy as well as combination strategies.50 In clinical trials evaluating cancer-testis antigen-targeted therapies, a single antigen formulation has been utilized most frequently. Herein, we described a coordinated expression of multiple cancer-testis antigens in the majority of myxoid and round cell liposarcomas, which supports the development of a polyvalent immunotherapeutic approach. Furthermore, the homogenous distribution of PRAME and NY-ESO-1 expression, which suggests clonal cancer-testis antigen activation, may result in an increased efficacy of cancer-testis antigen-targeted therapies in this particular neoplasm.6, 22, 23, 51 Of note, cancer-testis antigen expression appears to be secondary to promoter hypomethylation, and aberrant tumor cell methylation is also the likely mechanism for the coordinated expression of multiple cancer-testis antigens.25, 52, 53, 54
In conclusion, PRAME, CTAG2 (LAGE-1), and CTAG1B (NY-ESO-1) are expressed in the majority of myxoid and round cell liposarcomas with frequent homogenous distribution demonstrated by immunohistochemistry. Our results support a correlated expression of cancer-testis antigen mRNA and protein as well as a coordinated expression of multiple cancer-testis antigens. Overall, these findings further support the development of cancer-testis antigen-targeted immunotherapies in myxoid and round cell liposarcomas.
References
Toro JR, Travis LB, Wu HJ et al. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978-2001: an analysis of 26,758 cases. Int J Cancer 2006;119:2922–2930.
Patel SR, Burgess MA, Plager C et al. Myxoid liposarcoma. Experience with chemotherapy. Cancer 1994;74:1265–1269.
Hofmann O, Caballero OL, Stevenson BJ et al. Genome-wide analysis of cancer/testis gene expression. Proc Natl Acad Sci USA 2008;105:20422–20427.
Chen YT, Chiu R, Lee P et al. Chromosome X-encoded cancer/testis antigens show distinctive expression patterns in developing gonads and in testicular seminoma. Hum Reprod 2011;26:3232–3243.
Scanlan MJ, Gure AO, Jungbluth AA et al. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol Rev 2002;188:22–32.
Caballero OL, Chen YT . Cancer/testis (CT) antigens: potential targets for immunotherapy. Cancer Sci 2009;100:2014–2021.
Ludwig Institute for Cancer Research & Laboratorio Nacional de Computacao Cientifica. CTDatabasehttp://www.cta.lncc.br/(accessed 18 June 2013).
Suyama T, Shiraishi T, Zeng Y et al. Expression of cancer/testis antigens in prostate cancer is associated with disease progression. Prostate 2010;70:1778–1787.
Gure AO, Chua R, Williamson B et al. Cancer-testis genes are coordinately expressed and are markers of poor outcome in non-small cell lung cancer. Clin Cancer Res 2005;11:8055–8062.
Andrade VC, Vettore AL, Felix RS et al. Prognostic impact of cancer/testis antigen expression in advanced stage multiple myeloma patients. Cancer Immun 2008;8:2.
Xu H, Gu N, Liu ZB et al. NY-ESO-1 expression in hepatocellular carcinoma: a potential new marker for early recurrence after surgery. Oncol Lett 2012;3:39–44.
Bandic D, Juretic A, Sarcevic B et al. Expression and possible prognostic role of MAGE-A4, NY-ESO-1, and HER-2 antigens in women with relapsing invasive ductal breast cancer: retrospective immunohistochemical study. Croat Med J 2006;47:32–41.
Perez D, Herrmann T, Jungbluth AA et al. Cancer testis antigen expression in gastrointestinal stromal tumors: new markers for early recurrence. Int J Cancer 2008;123:1551–1555.
Szczepanski MJ, Deleo AB, Luczak M et al. PRAME expression in head and neck cancer correlates with markers of poor prognosis and might help in selecting candidates for retinoid chemoprevention in pre-malignant lesions. Oral Oncol 2013;49:144–151.
Taylor BJ, Reiman T, Pittman JA et al. SSX cancer testis antigens are expressed in most multiple myeloma patients: co-expression of SSX1, 2, 4, and 5 correlates with adverse prognosis and high frequencies of SSX-positive PCs. J Immunother 2005;28:564–575.
Santamaria C, Chillon MC, Garcia-Sanz R et al. The relevance of preferentially expressed antigen of melanoma (PRAME) as a marker of disease activity and prognosis in acute promyelocytic leukemia. Haematologica 2008;93:1797–1805.
Grau E, Oltra S, Martinez F et al. MAGE-A1 expression is associated with good prognosis in neuroblastoma tumors. J Cancer Res Clin Oncol 2009;135:523–531.
Ayyoub M, Taub RN, Keohan ML et al. The frequent expression of cancer/testis antigens provides opportunities for immunotherapeutic targeting of sarcoma. Cancer Immun 2004;4:7.
Skubitz KM, Cheng EY, Clohisy DR et al. Differential gene expression in liposarcoma, lipoma, and adipose tissue. Cancer Invest 2005;23:105–118.
Skubitz KM, Pambuccian S, Manivel JC et al. Identification of heterogeneity among soft tissue sarcomas by gene expression profiles from different tumors. J Transl Med 2008;6:23.
Segal NH, Blachere NE, Guevara-Patino JA et al. Identification of cancer-testis genes expressed by melanoma and soft tissue sarcoma using bioinformatics. Cancer Immun 2005;5:2.
Hemminger JA, Ewart Toland A, Scharschmidt TJ et al. The cancer-testis antigen NY-ESO-1 is highly expressed in myxoid and round cell subset of liposarcomas. Mod Pathol 2013;26:282–288.
Pollack SM, Jungbluth AA, Hoch BL et al. NY-ESO-1 is a ubiquitous immunotherapeutic target antigen for patients with myxoid/round cell liposarcoma. Cancer 2012;118:4564–4570.
Weiss SW, Goldblum JR . Liposarcoma In: Enzinger and Weiss’s Soft Tissue Tumors 5th edn Mosby Elsevier: Philadelphia, PA, USA, 2008, pp 477–516.
S Simpson AJ, Caballero OL, Jungbluth A et al. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer 2005;5:615–625.
Nicholaou T, Ebert L, Davis ID et al. Directions in the immune targeting of cancer: lessons learned from the cancer-testis AgNY-ESO-1. Immunol Cell Biol 2006;84:303–317.
Tyagi P, Mirakhur B . MAGRIT: the largest-ever phase III lung cancer trial aims to establish a novel tumor-specific approach to therapy. Clin Lung Cancer 2009;10:371–374.
Odunsi K, Matsuzaki J, Karbach J et al. Efficacy of vaccination with recombinant vaccinia and fowlpox vectors expressing NY-ESO-1 antigen in ovarian cancer and melanoma patients. Proc Natl Acad Sci USA 2012;109:5797–5802.
Robbins PF, Morgan RA, Feldman SA et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol 2011;29:917–924.
Rittig SM, Haentschel M, Weimer KJ et al. Intradermal vaccinations with RNA coding for TAA generate CD8+ and CD4+ immune responses and induce clinical benefit in vaccinated patients. Mol Ther 2011;19:990–999.
Peled N, Oton AB, Hirsch FR et al. MAGE A3 antigen-specific cancer immunotherapeutic. Immunotherapy 2009;1:19–25.
Morgan RA, Chinnasamy N, Abate-Daga D et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother 2013;36:133–151.
Ikeda H, Lethe B, Lehmann F et al. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity 1997;6:199–208.
Griffioen M, Kessler JH, Borghi M et al. Detection and functional analysis of CD8+ T cells specific for PRAME: a target for T-cell therapy. Clin Cancer Res 2006;12:3130–3136.
Weber JS, Vogelzang NJ, Ernstoff MS et al. A phase 1 study of a vaccine targeting preferentially expressed antigen in melanoma and prostate-specific membrane antigen in patients with advanced solid tumors. J Immunother 2011;34:556–567.
Ono T, Kurashige T, Harada N et al. Identification of proacrosin binding protein sp32 precursor as a human cancer/testis antigen. Proc Natl Acad Sci USA 2001;98:3282–3287.
Tammela J, Uenaka A, Ono T et al. OY-TES-1 expression and serum immunoreactivity in epithelial ovarian cancer. Int J Oncol 2006;29:903–910.
Quintarelli C, Dotti G, Hasan ST et al. High-avidity cytotoxic T lymphocytes specific for a new PRAME-derived peptide can target leukemic and leukemic-precursor cells. Blood 2011;117:3353–3362.
Atanackovic D, Luetkens T, Kloth B et al. Cancer-testis antigen expression and its epigenetic modulation in acute myeloid leukemia. Am J Hematol 2011;86:918–922.
Epping MT, Wang L, Edel MJ et al. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell 2005;122:835–847.
Lethe B, Lucas S, Michaux L et al. LAGE-1, a new gene with tumor specificity. Int J Cancer 1998;76:903–908.
McCormack E, Adams KJ, Hassan NJ et al. Bi-specific TCR-anti CD3 redirected T-cell targeting of NY-ESO-1- and LAGE-1-positive tumors. Cancer Immunol Immunother 2013;62:773–785.
van der Bruggen P, Traversari C, Chomez P et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 1991;254:1643–1647.
Ladelfa MF, Peche LY, Toledo MF et al. Tumor-specific MAGE proteins as regulators of p53 function. Cancer Lett 2012;325:11–17.
Tureci O, Sahin U, Schobert I et al. The SSX-2 gene, which is involved in the t(X;18) translocation of synovial sarcomas, codes for the human tumor antigen HOM-MEL-40. Cancer Res 1996;56:4766–4772.
Smith HA, McNeel DG . The SSX family of cancer-testis antigens as target proteins for tumor therapy. Clin Dev Immunol 2010;2010:150591.
Cheever MA, Allison JP, Ferris AS et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res 2009;15:5323–5337.
Chomez P, De Backer O, Bertrand M et al. An overview of the MAGE gene family with the identification of all human members of the family. Cancer Res 2001;61:5544–5551.
Gaugler B, Van den Eynde B, van der Bruggen P et al. Human gene MAGE-3 codes for an antigen recognized on a melanoma by autologous cytolytic T lymphocytes. J Exp Med 1994;179:921–930.
Pollack SM, Loggers ET, Rodler ET et al. Immune-based therapies for sarcoma. Sarcoma 2011;2011:438940.
Juretic A, Spagnoli GC, Schultz-Thater E et al. Cancer/testis tumour-associated antigens: immunohistochemical detection with monoclonal antibodies. Lancet Oncol 2003;4:104–109.
Glazer CA, Smith IM, Ochs MF et al. Integrative discovery of epigenetically derepressed cancer testis antigens in NSCLC. PloS ONE 2009;4:e8189.
De Smet C, Lurquin C, Lethe B et al. methylation is the primary silencing mechanism for a set of germ line- and tumor-specific genes with a CpG-rich promoter. Mol Cell Biol 1999;19:7327–7335.
Woloszynska-Read A, Mhawech-Fauceglia P, Yu J et al. Intertumor and intratumor NY-ESO-1 expression heterogeneity is associated with promoter-specific and global DNA methylation status in ovarian cancer. Clin Cancer Res 2008;14:3283–3290.
Acknowledgements
We thank Jessica Gillespie for performing the quantitative real-time PCR studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Modern Pathology website
Supplementary information
Rights and permissions
About this article
Cite this article
Hemminger, J., Toland, A., Scharschmidt, T. et al. Expression of cancer-testis antigens MAGEA1, MAGEA3, ACRBP, PRAME, SSX2, and CTAG2 in myxoid and round cell liposarcoma. Mod Pathol 27, 1238–1245 (2014). https://doi.org/10.1038/modpathol.2013.244
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2013.244
Keywords
This article is cited by
-
Myxoid Liposarcomas: Systemic Treatment Options
Current Treatment Options in Oncology (2023)
-
Immunotherapeutic Approaches to Sarcoma
Current Treatment Options in Oncology (2015)