Abstract
Molecular testing for mutations activating the mitogen-associated protein kinase signaling pathway is being used to help diagnose thyroid carcinomas. However, the prevalence of these mutations in thyroid lymphomas has not been reported. Therefore, we studied the prevalence of BRAF, NRAS, HRAS, and KRAS mutations in 33 thyroid lymphomas and correlated the mutational status with the clinical, pathological, cytogenetic, and immunophenotypic findings. Eleven cases were also tested for PAX8/PPARγ translocations. The lymphomas included 25 diffuse large B-cell lymphomas, 6 extranodal marginal-zone lymphomas of mucosa-associated lymphoid tissue type, and 2 follicular lymphomas. Seventeen diffuse large B-cell lymphomas were germinal center type, six non-germinal center type, and two unclassifiable (Hans algorithm). None of the cases had an associated thyroid carcinoma. Mutations of the BRAF gene were identified in six (24%) diffuse large B-cell lymphomas (D594G in three germinal center diffuse large B-cell lymphomas, K601N in two germinal center diffuse large B-cell lymphomas, and V600E in one non-germinal center diffuse large B-cell lymphoma) and of the NRAS gene in two (8%) non-germinal center diffuse large B-cell lymphomas (Q61K and Q61H). BRAF and NRAS mutations were not found in any extranodal marginal-zone lymphomas of mucosa-associated lymphoid tissue type or follicular lymphomas. HRAS and KRAS mutations were not identified in any of the cases, nor were PAX8/PPARγ translocations found. Thus, interpretation of finding a BRAF or NRAS mutation in the thyroid, particularly in preoperative thyroid aspirates, must take into account the differential diagnosis of a lymphoma. In addition to the diagnostic importance, our data also demonstrate that alteration in the mitogen-associated protein kinase pathway may have a role in the pathogenesis of some large B-cell lymphomas of the thyroid with potential therapeutic implications.
Similar content being viewed by others
Main
Papillary carcinoma is the most common type of thyroid cancer, representing approximately 80% of all malignant thyroid tumors.1 Primary thyroid lymphomas, on the other hand, are rare and account for 1–5% of all thyroid malignancies, and approximately 2% of all malignant extranodal lymphomas.2, 3, 4 Initial diagnosis of the epithelial thyroid neoplasms, and subsequent therapeutic planning, is often based on fine-needle aspiration biopsy and its cytological evaluation. To increase the diagnostic accuracy of thyroid fine-needle aspiration material, molecular testing for a panel of mutations is being more commonly used.5 Specifically, molecular analyses have focused on a set of somatic alterations of genes in the mitogen-activated protein kinase (MAPK) pathway, which are frequently present in carcinomas of the thyroid. These include point mutations of the BRAF and RAS genes, and RET/PTC and PAX8/PPARγ chromosomal rearrangements.6, 7, 8 V600E mutation in the BRAF gene has been identified as the most common genetic event in papillary thyroid carcinoma occurring in 40–45% of cases.9, 10 This mutation is also found in poorly differentiated and anaplastic thyroid carcinomas that typically have a component of residual well-differentiated papillary carcinoma.11 RAS mutations, on the other hand, are seen more commonly in thyroid tumors with a follicular pattern, including follicular carcinoma, follicular adenoma, and follicular variant of papillary carcinoma.5
Until the very recent discovery of BRAF V600E mutations in virtually all cases of hairy cell leukemia,12, 13, 14, 15, 16 the presence of these mutations in non-Hodgkin lymphomas had received little attention. The recent studies of BRAF mutations in hairy cell leukemia have also included a very large number of B-cell lymphomas and have identified only two cases of other chronic lymphoproliferative disorder, not fulfilling the criteria for hairy cell leukemia with BRAF V600E mutations. However, the presence of V600E mutation in these two chronic lymphoproliferative disorders could not be confirmed on Sanger sequencing.12, 13, 14, 16, 17 In an older study, Lee et al18 reported BRAF mutations in 6% (4/67) of diffuse large B-cell lymphomas involving various non-thyroid sites. However, all four of these BRAF mutations involved codons other than codon 600, where the most common mutation occurs in papillary thyroid carcinoma. Borie et al19 studied immunodeficiency-related non-Hodgkin lymphomas with microsatellite instability and found V600E BRAF mutations in three of their nine cases, which included one diffuse large B-cell lymphoma, one T-cell post-transplant lymphoproliferative disorder, and one primary central nervous system B-cell non-Hodgkin lymphoma. V600E BRAF mutations have also been reported in more than half of Langerhans cell histiocytoses,20 rare T-cell acute lymphoblastic leukemias,21 and few cases of multiple myeloma.22 In addition, NRAS and KRAS mutations have frequently been reported in plasma cell myeloma and plasma cell leukemias,23, 24 B-cell acute lymphoblastic leukemias,21 and some cutaneous T-cell lymphomas.25 The frequency of these mutations in lymphomas of the thyroid, where they might cause the greatest diagnostic confusion, is unknown.
We recently encountered a thyroid mass sampled by fine-needle aspiration, where a cytological diagnosis of malignant tumor was rendered with a differential diagnosis that included both carcinoma and lymphoma. Molecular analysis was positive for a BRAF V600E (c. 1799T>A) mutation, which was thought to support the diagnosis of thyroid carcinoma. The tumor was excised and found to represent a diffuse large B-cell lymphoma with no evidence of a papillary carcinoma in the thyroid gland. This case prompted us to perform a systematic study of BRAF and other common mutations activating the MAPK pathway (NRAS, KRAS, and HRAS) in 33 lymphomas of the thyroid. Eleven cases were also investigated for PAX8/PPARγ rearrangement.
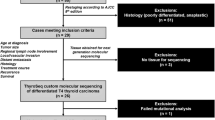
Materials and methods
Case Selection and Review
A total of 33 B-cell lymphomas presenting in the thyroid were obtained from the Department of Pathology at the University of Pittsburgh. The study was approved by the University of Pittsburgh Institutional Review Board. The following clinical features were recorded after review of the de-identified information from electronic medical records: gender, age, clinical presentation, treatment, and follow-up.
The routine histological sections and cytological preparations, together with all available immunohistochemical stains, flow cytometric data, and cytogenetic findings were reviewed (NA, SHS). When not already available, at least the following immunohistochemical stains for the following antigens were performed: CD20, CD3, kappa, lambda, CD10, BCL6, IRF4/MUM1, Ki-67, Cyclin D1, and PAX8 (Table 1). Lymphomas were classified using the WHO criteria,26 and diffuse large B-cell lymphoma further characterized using the Hans’ algorithm into germinal center (CD10+ or BCL6+ and IRF4/MUM1−) and non-germinal center (CD10−, BCL6− or BCL6+, IRF4/MUM1+) types.27 Results of all available classical cytogenetic or fluorescence in-situ hybridization studies were also reviewed.
Microdissection and Nucleic Acid Isolation
Histological sections with the most dense lymphoma infiltration were identified, and 5-μm sections cut for BRAF and RAS mutation analysis. The targeted areas were manually microdissected to collect tumor tissue for DNA isolation. In one case, two different areas representing extranodal marginal-zone lymphoma of mucosa-associated lymphoid tissue type and diffuse large B-cell lymphoma were microdissected separately. Genomic DNA was isolated using the DNeasy Blood and Tissue kit (Qiagen, Valencia, CA, USA), according to the manufacturer's instructions. Total nucleic acid was isolated from snap-frozen tissue in 11 available cases (8 diffuse large B-cell lymphoma, germinal center type; 2 diffuse large B-cell lymphoma, non-germinal center type, and 1 extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue type) using the MagNA Lyser and MagNA Pure (Roche) for PAX8/PPARγ rearrangement studies.
Detection of Point Mutations
First, mutational analysis was performed for hotspots typically found in thyroid cancer, ie, BRAF codons 600 and 601, KRAS codons 12 and 13, HRAS codon 61, and NRAS codon 61, using real-time LightCycler PCR followed by fluorescence melting-curve analysis as previously described.6 Briefly, for each gene, a pair of oligonucleotide primers flanking the mutation site was designed together with two fluorescent probes with the sensor probe spanning the codon of interest (TIB Molbiol, Berlin, Germany). Amplification was performed for 40 cycles. Post-amplification fluorescence melting-curve analysis was performed by gradual heating of samples at a rate of 0.1 C/sec from 45 to 95 C. For each mutation hotspot, DNA from a tumor or cell line known to carry a specific mutation was used as a positive control, and DNA from peripheral blood lymphocytes was used as a wild-type negative control. The sensitivity of mutation detection by the melting-curve analysis was 10% of cells with a mutant allele in the background of normal cells, as established by serial dilutions of the positive controls. Those samples that revealed no mutations in these hotspots were further analyzed for mutations in the entire exon 15 of the BRAF gene, exon 2 of NRAS and HRAS, and exon 1 of KRAS, using Sanger sequencing. Specifically, PCR amplification was performed using 25 ng of DNA and AmpliTaq Gold PCR Master Mix (Applied Biosystems, Foster City, CA, USA). The PCR products were sequenced using the BigDye Terminator v3.1 Cycle Sequencing kit (Applied Biosystems).
Detection of PAX8/PPARγ Rearrangement by RT-PCR
In those samples in which frozen tissue was available, PAX8/PPARγ rearrangement was detected by RT-PCR as previously described.6 Briefly, reverse transcription and PCR amplification were performed in one step using QuantiTech Probe RT-PCR Kit (Qiagen), and gene-specific primers and probes. The reverse transcription was carried out at 50 C for 30 min, followed by 40-cycle PCR amplification. RNA from a tumor known to carry the PAX8/PPARγ rearrangement was used as a positive control. The sensitivity of mutation detection by RT-PCR was 1% of cells carrying the rearrangement in the background of normal cells, as established by serial dilutions of the positive control.
Cytogenetic Studies
Classical G-banded cytogenetic karyotypes were obtained using previously described methods.28 Fluorescence in-situ hybridization was performed on interphase nuclei according to the manufacturer's protocol, using IGH/MYC dual-color translocation probe, IGH break-apart probe, and MYC break-apart probe (Vysis DNA probes, Abbott Molecular, Des Plains, IL, USA). Two hundred nuclei were analyzed for each of the probes. The cut-off value used for false-positive signals for the translocation probe was 1%, and for the break-apart probe was 3%.29
Results
The patients included 28 females and 5 males with a mean age of 65 years (range of 30–94 years). All patients presented with a neck mass and a clinical diagnosis of a thyroid tumor. Biopsies/excisions showed 25 diffuse large B-cell lymphoma with 17 of germinal center type, 6 of non-germinal center type, and 2 unclassifiable (Hans’ algorithm), 6 extranodal marginal-zone lymphomas of marginal-zone lymphoid tissue, including 3 with extensive plasmacytic differentiation, and 2 follicular lymphomas (one grade 1–2 of 3 and the other grade 3A of 3). None of the cases revealed an associated thyroid carcinoma based on histopathological and immunohistological studies. However, in addition to staining of thyroid epithelium, more than 80% neoplastic cells were positive for PAX8 in 26/33 lymphomas, consistent with a recent study reporting that this antibody cross-reacts with PAX5.30 Most of the plasmacytic areas of the tumors were PAX8-negative.
The index patient had a thyroid mass that was rapidly enlarging and was clinically suspected to be a carcinoma or lymphoma. Fine-needle aspiration biopsy of the lesion showed numerous large neoplastic cells with scant to moderate amount of cytoplasm, round to somewhat irregular nuclear contours, and conspicuous nucleoli in a background of small lymphocytes (Figure 1a). A part of the specimen was sent for flow cytometric immunophenotypic evaluation and another for molecular testing. The flow cytometric studies revealed polyclonal small B lymphocytes and admixed T cells; however, they did not characterize the large atypical cells. Molecular analysis revealed a BRAF V600E (c. 1799T>A) mutation (Figure 1b). The case was diagnosed as a malignant neoplasm favoring an anaplastic thyroid carcinoma. The thyroidectomy was performed and pathological examination revealed a CD20-positive diffuse large B-cell lymphoma of non-germinal center type (Figure 1c–e). The thyroid gland was entirely sectioned and submitted for microscopic examination, which revealed no papillary or anaplastic carcinoma. The mutation analysis was repeated on cells isolated from four different areas of the lymphoma, all of which confirmed the presence of a BRAF V600E mutation. The adjacent normal thyroid tissue revealed wild-type BRAF. The patient was subsequently treated with CHOP-R chemoimmunotherapy (cyclophosphamide, vincristine, adriamycin, and prednisone with rituximab) and radiation following his surgery. The patient is alive and had no evidence of disease on PET-CT scan 2 years post-diagnosis.
Diffuse large B-cell lymphoma, index case. (a) The fine-needle aspiration biopsy demonstrates numerous large cells with scant cytoplasm, round to somewhat irregular nuclei and admixed small lymphocytes. (Papanicolaou stain, original magnification × 1000) (b) Sequencing of exon 15 of the BRAF gene revealed a 1799T>A, V600E mutation. (c) The excision demonstrated a diffuse proliferation of large transformed cells (hematoxylin and eosin, original magnification × 1000). (d) The neoplastic cells were CD20-positive (original magnification, × 1000) (e) with a relatively high Ki-67 proliferative index (original magnification, × 200).
Molecular analysis of the 33 total thyroid lymphomas revealed 8 (24%) cases positive for one of the studied mutations. All mutations were identified in diffuse large B-cell lymphoma (8/25, 32%; Table 2). There were six BRAF mutations, including the BRAF V600E (c.1799T>A) mutation found in the diffuse large B-cell lymphoma, non-germinal center index case, three D594G (c.1781A>G) mutations (Figure 2a), and two K601N (Figure 2b) mutations, all in diffuse large B-cell lymphoma, germinal center type. Two of the non-germinal center type diffuse large B-cell lymphoma had NRAS mutations, both at codon 61, Q61K, and Q61H (Figure 3). There was no statistically significant difference between the frequencies of mutation in germinal center vs non-germinal center diffuse large B-cell lymphoma. One case of diffuse large B-cell lymphoma also showed a low-grade area of extranodal marginal-zone lymphoma of mucosa-associated lymphoid tissue type, which was separately analyzed for the mutations. Both areas (diffuse large B-cell lymphoma and extranodal marginal-zone lymphoma of mucosa-associated lymphoid tissue type) were negative for mutations. None of the cases showed mutations of KRAS codons 12/13 or HRAS codon 61(0/33) or PAX8/PPARγ rearrangement (0/11).
Classical cytogenetic studies demonstrated complex structural and numerical abnormalities in 7/12 diffuse large B-cell lymphoma cases tested (Table 3). No translocation t(2;3)(q13;p25) that corresponds to PAX8/PPARγ rearrangements was observed. No apparent abnormalities were seen in 7q34 (BRAF), 1p13.2 (NRAS), 12p12.1 (KRAS), or 11p15.5 (HRAS). Two cases showed IGH/MYC translocation by fluorescence in-situ hybridization, one of which also had classical cytogenetics that showed a t(8;14) translocation.
Treatment information was available for 30 cases. Twenty-five patients received definitive surgery (nine received surgery alone, five received surgery with chemotherapy, eight received surgery with radiation, and three received surgery, radiation, and chemotherapy), four patients received chemoimmunotherapy alone, and one patient received chemotherapy and radiation. Chemoimmunotherapy was CHOP-R in all cases except for one patient who was given a Vanderbilt regimen. Four patients had a past history of lymphoma. Two patients developed diffuse large B-cell lymphoma, germinal center type of the thyroid following a prior lymphoma of unspecified type (one renal, one gastric). The other two patients had a prior history of diffuse large B-cell lymphoma, one in the left lobe of thyroid, and the other of the mediastinum. Both underwent surgery and received chemoradiation, and then presented with an extranodal marginal-zone lymphoma of mucosa-associated lymphoid tissue type of the right thyroid lobe.
Eleven (11/33, 33%) patients died after their initial diagnosis of thyroid lymphoma. All patients diagnosed with either extranodal marginal-zone lymphoma of mucosa-associated lymphoid tissue type or follicular lymphoma were alive after a median follow-up of 3.5 years. In all, 4/8 (50%) patients with diffuse large B-cell lymphoma with mutations and 7/17 (41%) patients with diffuse B-cell lymphoma without mutations died. There was no statistically significant difference in overall survival between the two groups (P=0.77; Figure 4).
Discussion
We report here for the first time the occurrence of BRAF and NRAS mutations in thyroid lymphomas. None of the cases studied had a coexistent thyroid carcinoma. It is also important to recognize that at least one commonly used antibody for PAX8, which may be helpful in confirming the follicular thyroid cell origin,31 cross-reacts with PAX5, and thus stains many B-cell lymphomas.30
All eight of these MAPK signaling pathway-activating mutations were identified in diffuse large B-cell lymphoma, with none found in the extranodal marginal-zone lymphoma of the mucosa-associated lymphoid tissue type or follicular lymphoma, although the number of these more indolent lymphomas was relatively small. There was no difference in the incidence of these mutations between germinal center and non-germinal center subgroups of diffuse large B-cell lymphoma. However, these two subgroups have been shown to be different, genetically and clinically, and the germinal center group has been reported to have a better prognosis.32
The MAPK cascade controls a major signaling network involved in various cellular functions. The signal is propagated through the RAS-RAF-MEK-ERK proteins into the nucleus regulating cell proliferation, survival, and differentiation.33 Constitutive activation of the effectors of this signaling pathway has a critical role in thyroid carcinogenesis. RET/PTC, RAS, and BRAF mutations appear to be mutually exclusive in thyroid carcinomas, suggesting that activation of this pathway at one level is sufficient for carcinogenesis.10
Molecular testing for alterations in the MAPK pathway genes is becoming an important part of the routine evaluation of fine-needle aspiration samples obtained from thyroid nodules. It is considered reliable and feasible, and has been shown to improve the overall accuracy of fine-needle aspiration cytology.6, 34, 35 A prospective evaluation of 470 fine-needle aspiration samples showed that molecular testing was particularly informative for cases in the indeterminate cytology category, where molecular testing increased the probability of carcinoma from 40% to close to 100% in cases in which mutations were identified.6 Detection of BRAF mutation was reported to have close to 100% positive predictive value for papillary thyroid carcinoma, and the risk of malignancy is above 80% when RAS mutation is detected.5 The importance of the diagnostic use of molecular markers has been reflected in the revised American Thyroid Association's management guidelines, which recommends the use of the mutational panel for nodules with indeterminate fine-needle aspiration cytology to help guide clinical management.36 In view of the increasing use of molecular testing in thyroid fine-needle aspiration samples, the specificity of these mutations becomes an important diagnostic and clinical issue.
The BRAF gene encodes a cytoplasmic serine/threonine kinase that is regulated by binding of RAS and propagates signals downstream to MEK kinase along the mitogen-associated protein kinase signaling pathway. Virtually, all BRAF mutations reported to date are located within the activation segment domain or G-loop, with the most common mutation being V600E (c.1799T>A).37 V600E BRAF mutation is the most common genetic event in papillary thyroid carcinoma, where it is found in 40–45% of cases.38 It is also found in poorly differentiated and anaplastic thyroid carcinomas, but not in medullary carcinoma, follicular tumors, or benign hyperplastic nodules.11
Although screening of human tumors for BRAF mutations has been widely performed, the data concerning the frequency and type of mutations in lymphomas are very limited. Lee et al18 detected BRAF mutations in 4/164 (2.4%) non-Hodgkin lymphomas. All four cases were diffuse large B-cell lymphoma, and no mutations were identified in other non-Hodgkin lymphoma analyzed (3 mantle cell lymphomas, 4 follicular lymphomas, 49 extranodal marginal-zone lymphoma of mucosa-associated lymphoid tissue type, and 34 T-cell lymphomas). Whereas most BRAF mutations in human cancers, including carcinomas of the thyroid, involve V600E, all four BRAF mutations reported in this study involved other amino acids. These included one G469A, two G469R, and one D594G (previously reported as G468A, G468R, and D593G, respectively). V600E BRAF mutation has been observed in immunodeficiency-related non-Hodgkin lymphoma with microsatellite instability. The BRAF mutation in this subset of patients was not restricted to diffuse large B-cell lymphoma, but was also seen in a T-cell post-transplant lymphoproliferative disorder and a primary central nervous system B-cell non-Hodgkin lymphoma.19 BRAF V600E mutations have also been identified in over half of Langerhans cell histiocytoses20 and in all hairy cell leukemias.12, 13, 14, 15, 16 Recent studies have also identified BRAF mutations, including V600E and non-V600E mutations, in a small number of plasma cell myelomas (9/238 with 4 V600E mutation),14, 22 and some acute lymphoblastic leukemias (2/3 T-acute lymphoblastic leukemias with 1 V600E and 4/25 B-acute lymphoblastic leukemias with no V600E).21 BRAF mutations other than V600E have also been identified in chronic lymphocytic leukemias17 and one splenic marginal-zone lymphoma.13 In contrast, however, other than the two cases of chronic lymphoproliferative disorder described by Arcaini et al12, several recent large studies of BRAF V600E mutations in hairy cell leukemia have not found any other B-cell lymphomas with V600E mutations.12, 13, 14, 15, 16
In the current study, BRAF mutations were found in 6/25 diffuse large B-cell lymphomas. The index case had a V600E BRAF mutation, the type typically associated with papillary thyroid carcinoma. The other mutations in the BRAF gene that were found (D594G and K601N) have not been found in thyroid carcinomas. The most commonly identified mutation, present in three cases was D594G (c.1781A>G). It has been previously identified in diffuse large B-cell lymphoma,18 sporadic colorectal adenocarcinoma,39 and malignant melanoma.40 A different mutation at the same site (D594N) has been reported in a plasma cell myeloma.14 The K601N mutation has been reported in colorectal adenocarcinoma and plasma cell myeloma.22, 39 A K601E mutation has been identified in one case of splenic marginal-zone lymphoma.13
RAS proteins are a molecular switch involved in propagating signals along several signaling pathways, including the MAPK and PI3K pathways. Approximately 10–20% of all human tumors have mutated RAS proteins.41 Activating point mutations of the RAS genes occur predominantly in epithelial thyroid neoplasms with a follicular pattern, including follicular adenomas and carcinomas, and the follicular variant of papillary carcinoma.5 They are also seen in poorly differentiated and anaplastic thyroid carcinomas.42, 43, 44 RAS gene mutations are reported in a small number of non-Hodgkin lymphoma,45 cutaneous T-cell lymphomas,25 and in up to about half of plasma cell neoplasms.23, 24 In this study, we identified NRAS mutations in two diffuse large B-cell lymphomas, with the overall prevalence of this mutation of 8% in thyroid diffuse large B-cell lymphoma. Both mutations (Q61H and Q61K) involved codon 61, which is the most common mutation site in this gene.46 The Q61K mutation is commonly seen in thyroid follicular carcinoma46 and in other malignancies such as melanoma.47 The Q61H mutation has also been reported in some thyroid carcinomas48 and papillary carcinoma of the breast.49
In summary, our results indicate that among primary thyroid tumors, BRAF and NRAS mutations are not restricted to epithelial neoplasms and may also be seen in thyroid lymphomas, specifically in diffuse large B-cell lymphomas. Importantly, mutations of these genes may be identical to those frequently seen in thyroid carcinomas, specifically V600E BRAF and Q61K NRAS mutations. Hence, when these mutations are detected in thyroid fine-needle aspiration samples, the differential diagnosis must also include lymphomas. Additional morphological and immunophenotypic studies remain critical in this situation to determine the correct diagnosis. Furthermore, our findings highlight the potential importance of the MAPK pathway mutations in the pathogenesis of diffuse large B-cell lymphomas developing in the thyroid gland.
References
Hundahl SA, Fleming ID, Fremgen AM, et al. A National Cancer Data Base report on 53 856 cases of thyroid carcinoma treated in the US, 1985–1995 (see comments). Cancer 1998;83:2638–2648.
Ansell SM, Grant CS, Habermann TM . Primary thyroid lymphoma. Semin Oncol 1999;26:316–323.
Honing ML, Seldenrijk CA, de Maat CE . Primary thyroid lymphoma. Neth J Med 1998;52:75–78.
Pedersen RK, Pedersen NT . Primary non-Hodgkin's lymphoma of the thyroid gland: a population based study. Histopathology 1996;28:25–32.
Nikiforov YE . Molecular diagnostics of thyroid tumors. Arch Pathol Lab Med 2011;135:569–577.
Nikiforov YE, Steward DL, Robinson-Smith TM, et al. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J Clin Endocrinol Metab 2009;94:2092–2098.
Cantara S, Capezzone M, Marchisotta S, et al. Impact of proto-oncogene mutation detection in cytological specimens from thyroid nodules improves the diagnostic accuracy of cytology. J Clin Endocrinol Metab 2010;95:1365–1369.
Ohori NP, Nikiforova MN, Schoedel KE, et al. Contribution of molecular testing to thyroid fine-needle aspiration cytology of ‘follicular lesion of undetermined significance/atypia of undetermined significance’. Cancer Cytopathol 2010;118:17–23.
Cohen Y, Xing M, Mambo E, et al. BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst 2003;95:625–627.
Kimura ET, Nikiforova MN, Zhu Z, et al. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res 2003;63:1454–1457.
Nikiforova MN, Kimura ET, Gandhi M, et al. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab 2003;88:5399–5404.
Arcaini L, Zibellini S, Boveri E, et al. The BRAF V600E mutation in hairy cell leukemia and other mature B-cell neoplasms. Blood 2012;119:188–191.
Blombery P, Wong SQ, Hewitt CA, et al. Detection of BRAF mutations in patients with hairy cell leukemia and related lymphoproliferative disorders. Haematologica 2011.
Boyd EM, Bench AJ, van’t Veer MB, et al. High resolution melting analysis for detection of BRAF exon 15 mutations in hairy cell leukaemia and other lymphoid malignancies. Br J Haematol 2011;155:609–612.
Tiacci E, Trifonov V, Schiavoni G, et al. BRAF mutations in hairy-cell leukemia. N Engl J Med 2011;364:2305–2315.
Tiacci E, Schiavoni G, Forconi F, et al. Simple genetic diagnosis of hairy cell leukemia by sensitive detection of the BRAF-V600E mutation. Blood 2012;119:192–195.
Zhang X, Reis M, Khoriaty R, et al. Sequence analysis of 515 kinase genes in chronic lymphocytic leukemia. Leukemia 2011;25:1908–1910.
Lee JW, Yoo NJ, Soung YH, et al. BRAF mutations in non-Hodgkin's lymphoma. Br J Cancer 2003;89:1958–1960.
Borie C, Colas C, Dartigues P, et al. The mechanisms underlying MMR deficiency in immunodeficiency-related non-Hodgkin lymphomas are different from those in other sporadic microsatellite instable neoplasms. Int J Cancer 2009;125:2360–2366.
Badalian-Very G, Vergilio JA, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood 2010;116:1919–1923.
Gustafsson B, Angelini S, Sander B, et al. Mutations in the BRAF and N-ras genes in childhood acute lymphoblastic leukaemia. Leukemia 2005;19:310–312.
Chapman MA, Lawrence MS, Keats JJ, et al. Initial genome sequencing and analysis of multiple myeloma. Nature 2011;471:467–472.
Corradini P, Ladetto M, Inghirami G, et al. N- and K-ras oncogenes in plasma cell dyscrasias. Leuk Lymphoma 1994;15:17–20.
Bezieau S, Devilder MC, Avet-Loiseau H, et al. High incidence of N and K-Ras activating mutations in multiple myeloma and primary plasma cell leukemia at diagnosis. Hum Mutat 2001;18:212–224.
Kiessling MK, Oberholzer PA, Mondal C, et al. High-throughput mutation profiling of CTCL samples reveals KRAS and NRAS mutations sensitizing tumors toward inhibition of the RAS/RAF/MEK signaling cascade. Blood 2011;117:2433–2440.
Swerdlow SH, Campo E, Harris NL, et al. (eds) WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press: Lyon, France, 2008.
Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004;103:275–282.
Cook JR, Shekhter-Levin S, Swerdlow SH . Utility of routine classical cytogenetic studies in the evaluation of suspected lymphomas: results of 279 consecutive lymph node/extranodal tissue biopsies. Am J Clin Pathol 2004;121:826–835.
Dewald GW, Ketterling WA, Wyatt WA, et al. Cytogenetic studies in neoplastic hematologic disorders. In: McClatchey KD (ed) Clinical Laboratory Medicine 2nd edn 2002, pp 658–685.
Moretti L, Medeiros LJ, Kunkalla K, et al. N-terminal PAX8 polyclonal antibody shows cross-reactivity with N-terminal region of PAX5 and is responsible for reports of PAX8 positivity in malignant lymphomas. Mod Pathol 2011;25:231–236.
Bishop JA, Sharma R, Westra WH . PAX8 immunostaining of anaplastic thyroid carcinoma: a reliable means of discerning thyroid origin for undifferentiated tumors of the head and neck. Hum Pathol 2011;42:1873–1877.
Lenz G, Staudt LM . Aggressive lymphomas. N Engl J Med 2010;362:1417–1429.
Mitin N, Rossman KL, Der CJ . Signaling interplay in Ras superfamily function. Curr Biol 2005;15:R563–R574.
Nikiforova MN, Nikiforov YE . Molecular diagnostics and predictors in thyroid cancer. Thyroid 2009;19:1351–1361.
Cohen Y, Rosenbaum E, Clark DP, et al. Mutational analysis of BRAF in fine needle aspiration biopsies of the thyroid: a potential application for the preoperative assessment of thyroid nodules. Clin Cancer Res 2004;10:2761–2765.
Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167–1214.
Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002;417:949–954.
Xing M . BRAF mutation in thyroid cancer. Endocr Relat Cancer 2005;12:245–262.
Wojcik P, Okon K, Osuch C, et al. BRAF mutations in sporadic colorectal carcinoma from polish patients. Pol J Pathol 2010;61:23–26.
Smalley KS, Xiao M, Villanueva J, et al. CRAF inhibition induces apoptosis in melanoma cells with non-V600E BRAF mutations. Oncogene 2009;28:85–94.
Bos JL . ras oncogenes in human cancer: a review. Cancer Res 1989;49:4682–4689.
Lemoine NR, Mayall ES, Wyllie FS, et al. High frequency of ras oncogene activation in all stages of human thyroid tumorigenesis. Oncogene 1989;4:159–164.
Namba H, Rubin SA, Fagin JA . Point mutations of ras oncogenes are an early event in thyroid tumorigenesis. Mol Endocrinol 1990;4:1474–1479.
Zhu Z, Gandhi M, Nikiforova MN, et al. Molecular profile and clinical-pathologic features of the follicular variant of papillary thyroid carcinoma. An unusually high prevalence of ras mutations. Am J Clin Pathol 2003;120:71–77.
Ahuja HG, Foti A, Bar-Eli M, et al. The pattern of mutational involvement of RAS genes in human hematologic malignancies determined by DNA amplification and direct sequencing. Blood 1990;75:1684–1690.
Hou P, Liu D, Shan Y, et al. Genetic alterations and their relationship in the phosphatidylinositol 3-kinase/Akt pathway in thyroid cancer. Clin Cancer Res 2007;13:1161–1170.
Eskandarpour M, Huang F, Reeves KA, et al. Oncogenic NRAS has multiple effects on the malignant phenotype of human melanoma cells cultured in vitro. Int J Cancer 2009;124:16–26.
Suarez HG, du Villard JA, Severino M, et al. Presence of mutations in all three ras genes in human thyroid tumors. Oncogene 1990;5:565–570.
Troxell ML, Levine J, Beadling C, et al. High prevalence of PIK3CA/AKT pathway mutations in papillary neoplasms of the breast. Mod Pathol 2010;23:27–37.
Acknowledgements
This work was supported by the NIH Grant CA88041.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Aggarwal, N., Swerdlow, S., Kelly, L. et al. Thyroid carcinoma-associated genetic mutations also occur in thyroid lymphomas. Mod Pathol 25, 1203–1211 (2012). https://doi.org/10.1038/modpathol.2012.73
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2012.73