Abstract
Signet-ring cell mesothelioma is uncommon and only two case reports have been published on this mesothelioma variant, both of which were initially misdiagnosed as signet-ring cell carcinoma. Herein are reported 23 signet-ring cell mesotheliomas that were investigated by immunohistochemistry, 12 of which were also studied by electron microscopy. Twenty-one of the cases originated in the pleura and two in the peritoneum. For comparison purposes and in order to determine the value of these techniques in the differential diagnosis of these tumors, seven cases of signet-ring cell lung adenocarcinoma were also studied. All signet-ring cell mesotheliomas were positive for calretinin, keratin 5/6, keratin 7, and mesothelin, 93% for podoplanin, and 91% for WT1; whereas, none reacted for MOC-31, CEA, TAG-72, CD15, TTF-1, napsin A, or CDX2. Among signet-ring cell lung adenocarcinomas, 100% were positive for keratin 7, CEA, and napsin A, 86% each for TTF-1 and TAG-72, 71% for CD15, and 14% for mesothelin, while all were negative for calretinin, keratin 5/6, WT1, podoplanin, and CDX2. After analyzing the results, it is concluded that the panels of markers used in the differential diagnosis of this mesothelioma variant should include those markers that are usually expressed in mesotheliomas (eg, calretinin, keratin 5/6, WT1, and podoplanin), broad-spectrum carcinoma markers that are frequently expressed in adenocarcinomas regardless of their site of origin (eg, MOC-31 and CEA), and organ-associated markers (eg, TTF-1 and napsin A for lung), which allow the site of origin of a metastatic adenocarcinoma to be established. Electron microscopy can be very useful as it permits the identification of characteristic ultrastructural mesothelioma and adenocarcinoma markers, and it also allows a better understanding of the morphologic features seen on routine light microscopy. Pathologists should be aware of this mesothelioma subtype as it can potentially be confused with other tumors that exhibit signet-ring features.
Similar content being viewed by others
Main
The finding of occasional vacuolated cells exhibiting signet-ring-like morphology is not rare in mesotheliomas; however, epithelioid mesotheliomas having a large number of such cells are relatively uncommon and have been subclassified as signet-ring cell mesotheliomas.1 Signet-ring cell carcinomas can arise in a wide variety of organs, including lung,2, 3, 4, 5, 6 stomach,7, 8 colon,8, 9, 10 breast,8, 11, 12 urinary bladder,13, 14, 15, 16, 17 pancreas,18 salivary glands,19 and prostate.20, 21, 22, 23, 24, 25, 26, 27, 28 The signet-ring configuration seen in adenocarcinomas has traditionally been associated with the accumulation of large amounts of intracytoplasmic mucin. It can also occur as a result of the formation of a true intracytoplasmic lumen with displacement of the nucleus toward the periphery of the cell, in which case, special stains for mucin are often negative, as in the case of signet-ring cell carcinomas of the prostate.20 Although the presence of mucin has been used to distinguish mesotheliomas from adenocarcinomas, examples of mesotheliomas producing mucin, including cases having cells with signet-ring morphology, have been documented.29, 30, 31 Because of all of this and the fact that the serosal membranes are one of the most common sites of metastasis for signet-ring cell adenocarcinomas regardless of their site of origin, mesotheliomas with signet-ring morphology can potentially be confused with adenocarcinomas exhibiting signet-ring features, especially when they are associated with myxoid stroma or when the biopsy material is limited and does not contain areas displaying a more conventional morphology. In addition, signet-ring cell carcinomas of the lung can occasionally involve the pleura with encasement of the lung and, clinically and radiologically, mimic mesothelioma.32 Even though mesotheliomas with signet-ring features are often mentioned in major publications and review articles on the pathology of mesotheliomas,1, 33, 34 to my knowledge, only two case reports in which the primary focus was on this variant of epithelioid mesothelioma have been published.31, 35 Given that a detailed investigation of a series of signet-ring cell mesotheliomas has yet to be published, the study of 23 such cases was undertaken and the results are herein reported. The value of both immunohistochemistry and electron microscopy in assisting in distinguishing these tumors from signet-ring cell adenocarcinomas of the lung, several cases of which are included for comparison purposes, is also discussed. To my knowledge, the ultrastructural features of a signet-ring cell adenocarcinoma of the lung have not previously been described.
Materials and methods
Twenty-three cases of epithelioid mesothelioma with signet-ring cell features were identified from the files of the Department of Pathology and the Electron Microscopy section at the University of Texas MD Anderson Cancer Center. The cases were selected based on at least 10% of the tumor being composed of cells exhibiting signet-ring-like morphology and they were then compared with seven cases of signet-ring cell adenocarcinoma of the lung. Tissue specimens were fixed in 10% buffered formalin and processed for routine light microscopy. Sections were cut and stained with hematoxylin-and-eosin in all cases. Mayer’s mucicarmine stain was done in selected cases. Immunohistochemical studies were performed on formalin-fixed, paraffin-embedded tissue sections using the streptavidin-biotinylated horseradish peroxidase complex method in a Dako AutoStainer (Carpinteria, CA, USA). The primary antibodies are listed in Table 1. The immunostaining was carried out using the LSAB2 peroxidase kit (Dako). To enhance the immunostaining, a heat epitope retrieval procedure was performed using a Black-and-Decker vegetable steamer (Shelton, CT, USA). Briefly, deparaffinized sections were placed in a thermoresistant container filled with a buffer solution. Depending upon the antibody, the buffer solution used was citrate buffer (pH 6.0) or a 1:1 solution of Tris-EDTA buffer (pH 8.0). The antigen–antibody immunoreaction was visualized using either 3-amino-9-ethylcarbazole or 3,3′-diaminobenzidine as chromogen. To evaluate the specificity of the immunoreaction, known positive and negative tissues were used as controls. The immunostaining was graded on a sliding scale of 1+ to 4+ according to the percentage of reactive cells (trace, <1%; 1+, 1–25%; 2+, 26–50%; 3+, 51–75%; 4+,>75%). Ultrastructural studies were performed on 12 of the mesothelioma cases and in 1 of the signet-ring cell adenocarcinomas of the lung. Tissue samples were fixed in 2% glutaraldehyde in phosphate buffer, post-fixed in 1% osmium tetroxide, and embedded in Epon epoxy resin. Ultrathin sections were stained with uranyl acetate and lead citrate.
Results
Clinical Findings
Twenty patients were men and 3 were women ranging in age from 43 to 74 years (mean, 60 years). There was a history of asbestos exposure in 12 patients and smoking in 16. The mesothelioma originated in the pleura in 21 cases and in the peritoneum in 2. Two of the patients (cases 19 and 21) were initially diagnosed as having signet-ring cell adenocarcinomas. Fifteen of the 21 patients with pleural mesothelioma underwent extrapleural pneumonectomy and most also received radiotherapy and/or chemotherapy after surgery (Table 2). Four underwent decortication and/or tumor debulking followed by radiotherapy and chemotherapy in two cases, chemotherapy alone in one, and radiotherapy, chemotherapy, and lobectomy in 1. One patient received radiotherapy and chemotherapy, and the remaining patients received chemotherapy alone. The two patients with peritoneal mesothelioma underwent tumor debulking followed by chemotherapy. Twenty-two patients died of disease 3–42 months (mean, 15 months) after diagnosis; no follow-up information was available in the remaining patient.
Pathology Findings
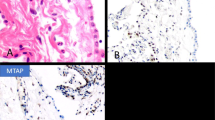
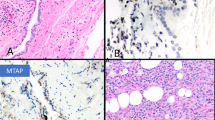
Gross examination of the 15 pneumonectomy specimens showed diffuse involvement of the visceral and parietal pleura with encasement of the lung. Five of the patients had involvement of the adjacent lung parenchyma, diaphragm, and pericardium (cases 12, 16, 17, 20, and 21), five of the adjacent lung parenchyma and diaphragm (cases 2, 6, 11, 13, and 14), one of the pericardium, diaphragm, and chest wall (case 5), one of the chest wall alone (case 7), and two of the adjacent lung parenchyma alone (cases 9 and 10). Multiple lymph nodes were involved by metastases in 11 of the cases (cases 5, 6, 7, 9, 11, 12, 13, 14, 16, 17, and 21). The most significant microscopic findings are summarized in Table 2. In all of the biopsy specimens, the tumor appeared to be largely composed of epithelioid cells having signet-ring-like features. In the 15 patients who subsequently underwent extrapleural pneumonectomy, the tumor in the pneumonectomy specimens was classified as epithelioid in 13 cases and as biphasic in 2. In all instances, the tumor exhibited areas in which it appeared to be extensively composed of cells displaying signet-ring cell morphology. The signet-ring cells typically contained a large, intracytoplasmic, clear vacuole that displaced the nucleus toward the periphery of the cell (Figure 1a). The nuclei were often distorted and variably indented, and frequently exhibited a sickle or crescent shape (Figure 1b). Many of the cells showed early vacuolization that appeared to progress to a large vacuole that eventually occupied most of the cell (Figure 1c and d). Although most of the cells contained only a single nucleus, on occasion, two or more nuclei were seen (Figure 1e). In some instances, the vacuoles of adjacent signet-ring cells appeared to coalesce to form larger vacuoles, sometimes resulting in a lipomatous-like (Figure 1g and h), adenomatoid (Figure 2a–d), tubular (Figure 2e–h), or cystic-like pattern (Figure 3a–d). While the signet-ring cells had clear vacuoles in most cases, in some, the vacuoles contained variable amounts of a bluish granular material, which most probably represented proteoglycans (Figures 1f and 2b). This type of signet-ring cell was particularly numerous in two cases that had areas of prominent myxoid stroma. None of the seven cases stained with Mayer’s mucicarmine stain were positive.
Case 9. (a) Low magnification of an area in which most of the neoplastic cells exhibit prominent signet-ring-like features. (b) Higher magnification that better demonstrates the morphologic features of the signet-ring cells. The nuclei are often crescent shaped, hyperpycnotic, and located at the periphery of the cells. Case 11. (c) Low magnification of a lymph node involved by metastatic mesothelioma in which many of the neoplastic cells present signet-ring features. (d) Higher magnification in which many of the neoplastic cells exhibit early vacuolization that appears to progress to larger vacuoles. (e) Another area of the same case in which some of the signet-ring-like cells appear to be binucleated (inset) or forming tubular-like structures exhibiting peripherally located, hyperpycnotic nuclei (upper left and right). (f) In this area, the lumens of the signet-ring-like cells and the tubular-like structures contain abundant, flocculent, granular material, most probably proteoglycans, which could potentially be mistaken for mucin. Case 2. (g) Low magnification of an area in which the signet-ring-like cells appear to coalesce forming larger vacuoles that somewhat resemble fat cells. (h) Higher magnification showing some of the larger vacuoles separated by thin septa.
Case 23. (a) Low-power magnification of an area exhibiting an adenomatoid pattern with glandular-like structures and abundant signet-ring-like cells. (b) Higher magnification showing abundant granular, bluish material, most probably proteoglycans, within the lumina of the glandular-like structures, as well as within the signet-ring-like cells (lower left corner). (c) Higher magnification of an area of the tumor in which the signet-ring cells appear to coalesce to form cystic-like or glandular-like structures. (d) Another area in which large amounts of proteoglycans are seen in the neoplastic signet-ring cells and in the interstitium. Case 3. (e) Area showing large clusters of tumor cells exhibiting a signet-ring morphology. (f) In this area of the tumor, the stroma is myxoid and the neoplastic cells are arranged in a trabecular pattern. Some of the signet-ring cells are coalescing, forming tubular structures. (g) Lower magnification showing an area that is primarily composed of tumor cells arranged in a tubular pattern. (h) Higher magnification of the same area to better show the cell morphology.
Case 2. (a) Low-power magnification of an area with numerous vacuolated cells and small cystic structures. (b) Higher magnification showing that some of the small vacuoles appear to progress to larger vacuoles, some of which occupy most of the cytoplasm, imparting a signet-ring appearance to the cells. (c) Higher magnification showing irregular cystic structures, most probably formed as a result of the coalescence of vacuolated cells. (d) Immunohistochemical preparation showing strong nuclear and cytoplasmic positivity for calretinin. Some of the cystic areas are lined by flattened neoplastic cells (lower right corner). (e) Diffuse strong cytoplasmic positivity for keratin 5/6. (f) Immunostaining for mesothelin of a solid area showing early formation of intracytoplasmic lumens, some of which appear as globoid inclusions. Strong reactivity is also seen along the cell membrane.
The neoplastic cells of the signet-ring cell lung adenocarcinomas appeared to contain variable amounts of mucin that, in some instances, seemed to displace the nucleus toward the periphery of the cell (Figure 4a). In addition, the signet-ring-like cells in some of the cases appeared to contain a relatively large, intracytoplasmic vacuole (Figure 4d) that was better seen by immunostaining for keratin 7 (Figure 4e).
(a) Signet-ring cell carcinoma of the lung in which the nuclei appear to have been displaced toward the periphery of the cell by large amounts of mucin. (b) Immunohistochemical preparation for keratin 7 in the same case showing strong reactivity throughout the cytoplasm. (c) Neoplastic cells in the same case showing strong nuclear positivity for TTF-1. (d) Hematoxylin-and-eosin-stained section of another case of signet-ring lung carcinoma in which many of the neoplastic cells appear to contain a large intracytoplasmic vacuole. (e) Immunohistochemical preparation for keratin 7 demonstrating that the large intracytoplasmic vacuoles seen on hematoxylin-and-eosin stain most likely represent intracytoplasmic lumina. (f) Immunostaining for napsin A showing granular positivity mostly at the periphery of the neoplastic cells.
Immunohistochemistry
The immunohistochemical results are summarized in Table 3. The signet-ring cells in all 23 of the mesothelioma cases reacted for calretinin and the staining was both nuclear and cytoplasmic (Figure 3d). These cells were also positive for keratin 5/6, keratin 7, and mesothelin in all cases stained for these markers (Figure 3e). The reactivity for the latter marker typically occurred along both the cell membrane and that bordering the intracytoplasmic lumina (Figure 3f). Twenty (91%) of 22 and 14 (93%) of 15 cases were positive for WT1 and podoplanin, respectively, and the staining was nuclear (WT1) and membranous (podoplanin). All cases stained for MOC-31, CEA, TAG-72, CD15, TTF-1, napsin A, keratin 20, or CDX2 were negative for these markers.
All signet-ring cell lung adenocarcinomas stained for keratin 7, MOC-31, CEA, and napsin A, as well as six of the seven stained for TTF-1, were positive for these markers (Figure 4b, c). None of the cases investigated for keratin 5/6, keratin 20, calretinin, WT1, podoplanin, or CDX2 were positive for any of these markers.
Electron Microscopic Findings
Electron microscopic studies demonstrated that the signet-ring-like appearance of the neoplastic cells seen on light microscopy was primarily caused by the presence of a single, or sometimes multiple, intracytoplasmic lumina that, in early stages, did not cause the displacement of the nucleus (Figure 5a). As the lumens increased in size, however, they progressively displaced the nucleus toward the periphery of the cell, thus creating the characteristic signet-ring-like appearance (Figure 5b and c). The cells usually contained a single nucleus with a prominent nucleolus, but binucleation was not rare. The cell membrane lining the intracytoplasmic lumina was often covered by a variable number of long microvilli or, on occasion, by a layer of electron-dense material that most probably represented proteoglycans (Figure 6a and b). Electron microscopy also showed that, in some instances, the signet-ring cell morphology noted by light microscopy was not caused by the presence of intracytoplasmic lumina, but rather by a pronounced dilatation of the intercellular space between two adjacent cells that was not discernible by light microscopy. This type of signet-ring cell may appear to be either mononucleated or binucleated depending on whether both nuclei were present in the plane of section (Figure 7a–d). An interesting finding in two of the cases was the presence of extracellular tubular crystalloids that were found in the intercellular space in close association with the microvilli (Figure 7a and d).
(a) Low-power magnification electron micrograph in which the cells show early formation of intracytoplasmic lumina. The small lumen in the center right (arrow) appears to be filled with microvilli. (b) Signet-ring cell with a single, large intracytoplasmic lumen, which appears to displace the nucleus toward the periphery of the cell. (c) Signet-ring cell with several intracytoplasmic lumina that may coalesce to form a single large intracytoplasmic lumen. (a, × 4000; b, × 4400; c, × 5100).
(a) Two groups of two neoplastic cells each showing marked dilatation of the intercellular space. The cell junctions between the two cells are apparent in the group on the right (arrows). The cell membranes that border the intercellular space are covered by long microvilli. On light microscopy, cells like these may appear as binucleated cells having a signet-ring-like morphology. (b) Two cells exhibiting signet-ring cell features caused by pronounced dilatation of the intercellular space. This is better demonstrated in the cell on the right in which the intercellular junctions are evident (arrows). (c) Higher magnification of a cell in which the signet-ring-like morphology is caused by the dilatation of the intercellular space between two adjacent cells. Only a small portion of the cytoplasm of the second cell is seen in the upper portion of the figure. The cell junctions of the two cells are evident (arrows). (d) Signet-ring features caused by massive dilatation of the intercellular space. Despite some artifactual disruption, the cell junction between the two cells is apparent in the lower part of the figure (arrows). A group of tubular crystalloids surrounded by electron-dense material is also present in the intercellular space. At higher magnification, the crystalloids appear limited by a double membrane (inset). (a, × 5400; b, × 9300; c, × 11 500; d, × 10 000, inset, × 37 500).
Ultrastructural examination of the signet-ring cell adenocarcinoma of the lung demonstrated that the signet-ring morphology was primarily caused by the intracytoplasmic accumulation of a large number of mucin granules of moderate electron density (Figure 8a). In addition to the mucin granules, some of the cells occasionally exhibited an intracytoplasmic lumen, along which mucin granules appeared to align and empty into the lumen (Figure 8b and c).
Signet-ring cell lung adenocarcinoma. (a) The nucleus appears to be displaced toward the periphery of the cell by a large number of mucin granules of medium and low electron density. (Inset: higher magnification showing a better detail of the granules.) (b) The same case showing another signet-ring cell having an intracytoplasmic lumen and abundant mucin granules. (c) Higher magnification of a portion of a signet-ring cell in which the mucin granules seem to be aligned along the intracytoplasmic lumen and some appear to be emptying their content into the lumen. (a, × 11 000, inset, × 18 000; b, × 17 500; c, × 20 000).
Discussion
Characteristically, mesotheliomas can present a diverse array of cytomorphologic features and grow in a wide variety of histologic patterns. Based on their morphology, these tumors have been classified into four major histologic subtypes: epithelioid, sarcomatoid, mixed epithelioid and sarcomatoid (biphasic), and desmoplastic, the most common of which is epithelioid.33 Most epithelioid mesotheliomas exhibit a tubulopapillary, adenomatoid, or solid pattern; however, in rare instances, they may present other histologic patterns, including deciduoid,36, 37, 38 clear cell,39, 40 adenoid cystic,34 pleomorphic,41, 42 small cell,43, 44 rhabdoid,45 glomeruloid,46 oncocytoid,47 and signet-ring cell.31, 35 Although the finding of an occasional signet-ring cell is not rare in mesotheliomas, the presence of large areas primarily composed of this type of cell is relatively uncommon, as is demonstrated by the fact that only a very limited number of publications on mesotheliomas with signet-ring-like features have been published. In a review of the literature, I was able to find only two publications that focused primarily on signet-ring cell mesotheliomas, both of which were case reports and both of these cases were peritoneal mesotheliomas.31, 35 One report was the case of a 57-year-old woman with no history of asbestos exposure who presented with recurrent ascites that was initially diagnosed as a carcinoma because of the presence of signet-ring cells in the cytologic preparations.35 The second was the case of a 59-year-old man with a 30-year history of heavy asbestos exposure who presented with ascites containing malignant cells whose precise type could not be determined.31 As ultrasound studies revealed thickening of the gastric wall, the possibility of linitis plastica was raised. The patient died 3 months later, and at autopsy multiple tumor nodules were found throughout the omentum, mesentery, and diaphragm. The stomach wall was markedly thickened and infiltrated by tumor cells. Microscopically, the neoplastic cells had signet-ring-like features and contained neutral mucin as demonstrated by Mayer’s mucicarmine stain. Immunohistochemical studies, together with electron microscopy, established the diagnosis of mesothelioma. Two of the cases in the present series were also initially misdiagnosed as signet-ring cell adenocarcinomas.
Cell vacuolization, including the presence of signet-ring cells, is a frequent feature of mesotheliomas that has not been sufficiently emphasized. Intracytoplasmic lumina are, in my experience, often seen in most subtypes of mesotheliomas, including some uncommon variants, such as small cell44 and deciduoid.38 In many instances, however, because of their size, they may not be apparent on routine light microscopy, but they can be easily demonstrated by electron microscopy or by the use of immunohistochemical markers, such as mesothelin or podoplanin, which are commonly expressed along the limiting membrane of the intracytoplasmic lumen (Figure 3f).
The present study demonstrates that signet-ring cell morphology is often caused by the presence of a single enlarging lumen within the cytoplasm or by multiple intracytoplasmic lumina that coalesce to form a larger one that progressively displaces the nucleus toward the periphery of the cell, resulting in the characteristic signet-ring-like features seen on light microscopy. The finding of binucleated signet-ring cells is not an unexpected finding as binucleation is not rare in mesotheliomas. What is unexpected is that, in some instances, when the binucleated signet-ring cells seen on light microscopy are viewed by electron microscopy, it becomes clear that the signet-ring-like morphology is actually the result of a massive dilatation of the intercellular space with displacement of the nuclei toward the periphery of the cells. This mechanism in the formation of signet-ring cells has not, to my knowledge, previously been described in either mesotheliomas or any other type of signet-ring cell tumor.
The morphologic features of the single case of signet-ring cell lung adenocarcinoma that was studied by electron microscopy were demonstrated to be the result of the presence of intracytoplasmic lumina and/or a large number of mucin-containing granules. Although mucin granules were not seen in the signet-ring cell mesotheliomas, some of the cases showed variable amounts of electron-dense material, thought to be proteoglycans, along the limiting membrane of the intracytoplasmic lumina, as well as in the extracellular space, especially in those cases exhibiting prominent myxoid stroma. Positivity for neutral mucin in mesotheliomas, as demonstrated by Mayer’s mucicarmine stain or periodic acid-Schiff with diastase pretreatment, is uncommon and has been reported to occur in about 5% of the cases,30 including signet-ring cell mesotheliomas.31 This positivity has been attributed by some authors to the presence of large amounts of proteoglycans.30 An interesting ultrastructural finding in two of the signet-ring cell mesothelioma cases in the present study was the presence of tubular crystalloids associated with microvilli. A similar type of crystalloid was previously reported by Hammar et al in 1996 in four mucicarmine-positive mesothelioma cases.30 More recently, in a prospective ultrastructural study, crystalloid inclusions, including the type seen in the present study, were found in 9 (15%) of 59 epithelioid mesotheliomas, which indicates that crystalloids are not as rare a finding in mesotheliomas as was previously believed.47 It was also concluded that, when present, these crystalloids can serve as a helpful ultrastructural marker for assisting in the diagnosis of mesotheliomas because of their unique morphology.
Another important observation in the present investigation is that signet-ring cells have a significant role in the development of the patterns seen in some of the histologic subtypes that have been described in mesotheliomas. When these cells are numerous and coalescent, they may produce a pattern resembling that of a well-differentiated lipomatous lesion, as was demonstrated not only in this study but also in previous reports,48, 49 including that of a pericardial mesothelioma that was predominantly composed of vacuolated cells exhibiting features reminiscent of a well-differentiated lipoma-like liposarcoma.48 This investigation also demonstrates that the coalescence of the vacuolated cells, including the signet-ring cells, can have a role in the formation of a microcystic, macrotubular, or adenomatoid-like pattern.
The results of the present study demonstrate that signet-ring cell mesotheliomas maintain the immunophenotype seen in other, more conventional, types of epithelioid mesotheliomas, particularly the expression of keratin 7, keratin 5/6, calretinin, and mesothelin, which were found to be diffusely and strongly positive in all of the cases in which expression for these markers was investigated. Immunoreactivity for other positive mesothelioma markers (WT1 and podoplanin) was also frequent, but somewhat more variable, as these markers were not found to be expressed in all of the cases and the staining was sometimes focal. An interesting immunohistochemical finding in some of the mesothelioma cases that were stained for mesothelin or podoplanin was the demonstration of small intracytoplasmic lumina or globoid-like inclusions in the solid areas of the tumor in which it was difficult or impossible to discern intracytoplasmic lumina on routine hematoxylin-and-eosin-stained sections (Figure 3f). Electron microscopy studies showed that the globoid-like staining pattern was associated with the presence of small intracytoplasmic lumina filled with microvilli. All of the broad-spectrum carcinoma markers investigated in the present study (MOC-31, CEA, TAG-72, and CD15) were negative for mesothelioma.
Mesotheliomas with signet-ring cell features can be confused with a variety of other tumors with similar morphology that can involve the serosal membranes. One of the tumors with the greatest potential of being confused with mesotheliomas with signet-ring cell features is signet-ring cell adenocarcinoma of the lung metastatic to the pleura. In those instances in which this differential diagnosis arises, immunohistochemical studies for lung-associated markers, such as TTF-150 and napsin A,51 can greatly facilitate this distinction as these markers have been reported to be frequently expressed in lung adenocarcinomas, but absent in mesotheliomas.52, 53, 54 The percentage of TTF-1 expression reported in signet-ring cell lung adenocarcinomas has ranged from 80 to 100%.4, 6, 55, 56, 57 In a combined review of four large series, 94 (85%) of 110 such cases were shown to express TTF-1.4, 6, 55, 57 These findings are in agreement with the results of the present study in which six (86%) of seven signet-ring cell lung adenocarcinomas were positive for this marker. Napsin A is a recently available lung adenocarcinoma marker that, similar to TTF-1, has also been reported to be frequently expressed in these tumors (∼60–90%).51, 53, 58, 59, 60 All four of the signet-ring cell adenocarcinomas of the lung that were stained for napsin A in the present study exhibited strong positivity for this marker. To my knowledge, this is the first study on napsin A expression in signet-ring cell adenocarcinomas of the lung.
Other frequent sites of origin of carcinomas with signet-ring cell morphology are the gastrointestinal tract and breast. As these tumors can metastasize to the serosal membranes, they can potentially be confused with signet-ring cell mesotheliomas, particularly in small biopsies or cytology specimens. As CDX2 is a sensitive and relatively specific marker for intestinal differentiation that has been reported to be expressed in the majority of signet-ring cell carcinomas of the colon (90–100%)8, 61 and less frequently in the stomach (∼45%),8, 62 but not in mesotheliomas, immunostaining for this marker, especially when it is used in conjunction with other markers, such as keratin 20, which is frequently expressed in colonic (∼85%)10 and gastric (∼45%)10 signet-ring cell carcinomas, but usually absent in mesotheliomas, can be useful in assisting in discriminating signet-ring cell carcinomas of the gastrointestinal tract from signet-ring cell mesotheliomas. Signet-ring cell carcinomas of the breast can be distinguished from signet-ring cell mesotheliomas by the combined use of breast-associated markers, such as mammaglobin and gross cystic disease fluid protein-15, estrogen receptor, which has been reported to be frequently positive in signet-ring cell breast carcinomas, but negative in signet-ring cell mesotheliomas,8, 63, 64 and mesothelioma markers, such as WT1 and podoplanin, which are frequently expressed in mesotheliomas, but negative in breast carcinomas.65
Although signet-ring cell mesotheliomas can be confused with metastatic signet-ring cell carcinomas, it should be kept in mind that the presence of signet-ring cell morphology is not a feature that is exclusively seen in epithelial tumors, as other neoplasms, such as epithelioid hemangioendotheliomas66 and melanomas,67, 68, 69 can, on occasion, exhibit this morphology. When the latter tumors are included in the differential diagnosis, immunostaining for endothelial markers, such as CD31 or CD34, or melanoma markers, such as melan A or HMB-45, which are commonly expressed in epithelioid hemangioendotheliomas and melanomas, respectively, but which are absent in mesotheliomas, can assist in distinguishing between these malignancies and signet-ring cell mesotheliomas.65
In conclusion, pathologists should be aware that mesotheliomas can, on occasion, present prominent signet-ring-like features and that, because of this, they can potentially be confused with other tumors exhibiting similar morphology, particularly signet-ring cell carcinomas, as was shown in two previously published case reports, as well as in two of the mesotheliomas in the present series that were initially diagnosed as metastatic adenocarcinomas with signet-ring cell features. In those instances in which the differential diagnosis between signet-ring cell mesothelioma and metastatic signet-ring cell carcinoma is difficult, immunohistochemical studies can be very helpful when the panel of markers includes those that are commonly expressed in mesotheliomas, such as WT1, podoplanin, keratin 5/6, and calretinin, broad-spectrum carcinoma markers that are frequently expressed in adenocarcinomas regardless of their site of origin (ie, MOC-31 and CEA), and organ-associated markers, which allow the site of origin of a metastatic adenocarcinoma to be determined.65 Electron microscopy can also be very useful, not only because it permits the identification of ultrastructural mesothelioma markers, such as the presence of the long, slender microvilli that are characteristic of mesothelioma, and the mucin granules that are a feature of adenocarcinomas, but it also allows a better understanding of the morphologic features seen on routine light microscopy.
References
Hammar SP, Henderson DW, Klebe S et al. Neoplasms of the pleura In: Tomashefski JF, Cagle PT, Farver CF, Fraire AE, (eds) Dail and Hammar’s Pulmonary Pathology, Vol. II. Neoplastic Lung Diseases 3rd edn Springer Science+Business Media: New York, NY, 2008, pp 558–734.
Kish JK, Ro JY, Ayala AG et al. Primary mucinous adenocarcinoma of the lung with signet-ring cells: a histochemical comparison with signet-ring cell carcinomas of other sites. Hum Pathol 1989;20:1097–1102.
Hayashi H, Kitamura H, Nakatani Y et al. Primary signet-ring cell carcinoma of the lung: histochemical and immunohistochemical characterization. Hum Pathol 1999;30:378–383.
Merchant SH, Amin MB, Tamboli P et al. Primary signet-ring cell carcinoma of the lung: immunohistochemical study and comparison with non-pulmonary signet-ring cell carcinomas. Am J Surg Pathol 2001;25:1515–1519.
Hiraki A, Ueoka H, Yoshino T et al. Primary signet-ring cell carcinoma of the lung with histochemical characterization. Anticancer Res 2002;22:1079–1081.
Tsuta K, Ishii G, Nitadori J et al. Comparison of the immunophenotypes of signet-ring cell carcinoma, solid adenocarcinoma with mucin production, and mucinous bronchioloalveolar carcinoma of the lung characterized by the presence of cytoplasmic mucin. J Pathol 2006;209:78–87.
Wong PC, Ferenczy A, Fan LD et al. Krukenberg tumors of the ovary. Ultrastructural, histochemical and immunohistochemical studies of 15 cases. Cancer 1986;57:751–760.
Chu PG, Weiss LM . Immunohistochemical characterization of signet-ring cell carcinomas of the stomach, breast, and colon. Am J Clin Pathol 2004;121:884–892.
Tung SY, Wu CS, Chen PC . Primary signet ring cell carcinoma of colorectum: an age- and sex-matched controlled study. Am J Gastroenterol 1996;91:2195–2199.
Goldstein NS, Long A, Kuan SF et al. Colon signet ring cell adenocarcinoma: immunohistochemical characterization and comparison with gastric and typical colon adenocarcinomas. Appl Immunohistochem Mol Morphol 2000;8:183–188.
Frost AR, Terahata S, Yeh IT et al. The significance of signet ring cells in infiltrating lobular carcinoma of the breast. Arch Pathol Lab Med 1995;119:64–68.
Liu SM, Chen DR . Signet-ring cell carcinoma of the breast. Pathol Int 2000;50:67–70.
Braun EV, Ali M, Fayemi AO et al. Primary signet-ring cell carcinoma of the urinary bladder: review of the literature and report of a case. Cancer 1981;47:1430–1435.
Kondo A, Ogisu B, Mitsuya H . Signet-ring cell carcinoma involving the urinary bladder. Report of a case and review of 21 cases. Urol Int 1981;36:373–379.
Kitamura H, Sumikawa T, Fukuoka H et al. Primary signet-ring cell carcinoma of the urinary bladder. Report of two cases with histochemical studies. Acta Pathol Jpn 1985;35:675–686.
Bernstein SA, Reuter VE, Carroll PR et al. Primary signet-ring cell carcinoma of urinary bladder. Urology 1988;31:432–436.
Bodi I, Andrews TC, Howard RS et al. Carcinomatous meningitis from primary signet ring cell carcinoma of bladder. Histopathology 2004;44:394–396.
Marcy M, Chetaille B, Charafe-Jauffret E et al. [Signet ring cell carcinoma of the pancreas: a case report]. Ann Pathol 2002;22:314–316 French.
Ghannoum JE, Freedman PD . Signet-ring cell (mucin-producing) adenocarcinomas of minor salivary glands. Am J Surg Pathol 2004;28:89–93.
Ro JY, El-Naggar A, Ayala AG et al. Signet-ring-cell carcinoma of the prostate: electron-microscopic and immunohistochemical studies of eight cases. Am J Surg Pathol 1988;12:453–460.
Hejka AG, England DM . Signet ring cell carcinoma of prostate. Immunohistochemical and ultrastructural study of a case. Urology 1989;34:155–158.
Uchijima Y, Ito H, Takahashi M et al. Prostate mucinous adenocarcinoma with signet ring cell. Urology 1990;36:267–268.
Alline KM, Cohen MB . Signet-ring cell carcinoma of the prostate. Arch Pathol Lab Med 1992;116:99–102.
Smith C, Feddersen RM, Dressler L et al. Signet ring cell adenocarcinoma of prostate. Urology 1994;43:397–400.
Leong FJ, Leong AS, Swift J . Signet-ring carcinoma of the prostate. Pathol Res Pract 1996;192:1232–1238.
Randolph TL, Amin MB, Ro JY et al. Histologic variants of adenocarcinoma and other carcinomas of prostate: pathologic criteria and clinical significance. Mod Pathol 1997;10:612–629.
Kuroda N, Yamasaki I, Nakayama H et al. Prostatic signet-ring cell carcinoma: case report and literature review. Pathol Int 1999;49:457–461.
Fujita K, Sugao H, Gotoh T et al. Primary signet ring cell carcinoma of the prostate: report and review of 42 cases. Int J Urol 2004;11:178–181.
MacDougall DB, Wang SE, Zidar BL . Mucin-positive epithelial mesothelioma. Arch Pathol Lab Med 1992;116:874–880.
Hammar SP, Bockus DE, Remington FL et al. Mucin-positive epithelial mesotheliomas: a histochemical, immunohistochemical, and ultrastructural comparison with mucin-producing pulmonary adenocarcinomas. Ultrastruct Pathol 1996;20:293–325.
Cook DS, Attanoos RL, Jalloh SS et al. ‘Mucin-positive’ epithelial mesothelioma of the peritoneum: an unusual diagnostic pitfall. Histopathology 2000;37:33–36.
Guru PK, Phillips S, Ball MM et al. Pseudomesotheliomatous presentation of primary signet ring cell carcinoma of lung. Indian J Chest Dis Allied Sci 2005;47:209–211.
Churg A, Roggli V, Galateau-Salle F et al. Mesothelioma In: Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC, (eds) WHO Classification of Tumours. Pathology & Genetics. Tumours of the Lung, Pleura, Thymus and Heart. IARC Press: Lyon, France, 2004, pp 128–136.
Allen TC . Recognition of histopathologic patterns of diffuse malignant mesothelioma in differential diagnosis of pleural biopsies. Arch Pathol Lab Med 2005;129:1415–1420.
Rekhi B, Pathuthara S, Ajit D et al. ‘Signet-ring’ cells—a caveat in the diagnosis of a diffuse peritoneal mesothelioma occurring in a lady presenting with recurrent ascites: an unusual case report. Diagn Cytopathol 2010;38:435–439.
Nascimento AG, Keeney GL, Fletcher CD . Deciduoid peritoneal mesothelioma. An unusual phenotype affecting young females. Am J Surg Pathol 1994;18:439–445.
Ordóñez NG . Epithelial mesothelioma with deciduoid features: report of four cases. Am J Surg Pathol 2000;24:816–823.
Ordóñez NG . Deciduoid mesothelioma: report of 21 cases with review of the literature. Mod Pathol 2012, [Epub ahead of print].
Ordóñez NG, Myhre M, Mackay B . Clear cell mesothelioma. Ultrastruct Pathol 1996;20:331–336.
Ordóñez NG . Mesothelioma with clear cell features: an ultrastructural and immunohistochemical study of 20 cases. Hum Pathol 2005;36:465–473.
Kadota K, Suzuki K, Sima CS et al. Pleomorphic epithelioid diffuse malignant pleural mesothelioma: a clinicopathological review and conceptual proposal to reclassify as biphasic or sarcomatoid mesothelioma. J Thorac Oncol 2011;6:896–904.
Ordóñez NG . Pleomorphic mesothelioma: report of 10 cases. Mod Pathol 2012;25:1011–1022.
Mayall FG, Gibbs AR . The histology and immunohistochemistry of small cell mesothelioma. Histopathology 1992;20:47–51.
Ordóñez NG . Mesotheliomas with small cell features: report of eight cases. Mod Pathol 2012;25:689–698.
Ordóñez NG . Mesothelioma with rhabdoid features: an ultrastructural and immunohistochemical study of 10 cases. Mod Pathol 2006;19:373–383.
Martínez-Consuegra N, Muñoz-Juárez M, Ortiz-Hidalgo C . Unusual multifocal glomeruloid pattern in a well-differentiated papillary mesothelioma of the peritoneum. Int J Surg Pathol 2008;16:426–427.
Ordóñez NG . Mesotheliomas with crystalloid structures: report of nine cases, including one with oncocytic features. Mod Pathol 2012;25:272–281.
Shimazaki H, Aida S, Iizuka Y et al. Vacuolated cell mesothelioma of the pericardium resembling liposarcoma: a case report. Hum Pathol 2000;31:767–770.
Kobayashi S, Ida M, Matsui O et al. Lipomatous change in a brain metastasis from malignant pleural mesothelioma. Neuroradiology 2001;43:159–161.
Ordóñez NG . Value of thyroid transcription factor-1 immunostaining in tumor diagnosis: a review and update. Appl Immunohistochem Mol Morphol 2012;20:429–444.
Ordóñez NG . Napsin A expression in lung and kidney neoplasia: a review and update. Adv Anat Pathol 2012;19:66–73.
Ordóñez NG . Value of thyroid transcription factor-1, E-cadherin, BG8, WT1, and CD44S immunostaining in distinguishing epithelial pleural mesothelioma from pulmonary and nonpulmonary adenocarcinoma. Am J Surg Pathol 2000;24:598–606.
Amatya VJ, Takeshima Y, Kohno H et al. Caveolin-1 is a novel immunohistochemical marker to differentiate epithelioid mesothelioma from lung adenocarcinoma. Histopathology 2009;55:10–19.
Bishop JA, Sharma R, Illei PB . Napsin A and thyroid transcription factor-1 expression in carcinomas of the lung, breast, pancreas, colon, kidney, thyroid, and malignant mesothelioma. Hum Pathol 2010;41:20–25.
Castro CY, Moran CA, Flieder DG et al. Primary signet ring cell adenocarcinomas of the lung: a clinicopathological study of 15 cases. Histopathology 2001;39:397–401.
Rossi G, Murer B, Cavazza A et al. Primary mucinous (so-called colloid) carcinomas of the lung: a clinicopathologic and immunohistochemical study with special reference to CDX-2 homeobox gene and MUC2 expression. Am J Surg Pathol 2004;28:442–452.
Sentani K, Oue N, Tashiro T et al. Immunohistochemical staining of Reg IV and claudin-18 is useful in the diagnosis of gastrointestinal signet ring cell carcinoma. Am J Surg Pathol 2008;32:1182–1189.
Hirano T, Gong Y, Yoshida K et al. Usefulness of TA02 (napsin A) to distinguish primary lung adenocarcinoma from metastatic lung adenocarcinoma. Lung Cancer 2003;41:155–162.
Inamura K, Satoh Y, Okumura S et al. Pulmonary adenocarcinomas with enteric differentiation: histologic and immunohistochemical characteristics compared with metastatic colorectal cancers and usual pulmonary adenocarcinomas. Am J Surg Pathol 2005;29:660–665.
Terry J, Leung S, Laskin J et al. Optimal immunohistochemical markers for distinguishing lung adenocarcinomas from squamous cell carcinomas in small tumor samples. Am J Surg Pathol 2010;34:1805–1811.
De Lott LB, Morrison C, Suster S et al. CDX2 is a useful marker of intestinal-type differentiation: a tissue microarray-based study of 629 tumors from various sites. Arch Pathol Lab Med 2005;129:1100–1105.
Tian MM, Zhao AL, Li ZW et al. Phenotypic classification of gastric signet ring cell carcinoma and its relationship with clinicopathologic parameters and prognosis. World J Gastroenterol 2007;13:3189–3198.
Fadare O . Pleomorphic lobular carcinoma in situ of the breast composed almost entirely of signet ring cells. Pathol Int 2006;56:683–687.
Jones GE, Strauss DC, Forshaw MJ et al. Breast cancer metastasis to the stomach may mimic primary gastric cancer: report of two cases and review of literature. World J Surg Oncol 2007;5:75.
Ordóñez NG . Application of immunohistochemistry in the diagnosis of epithelioid mesothelioma: a review and update. Hum Pathol advance online publication, 7 September 2012 (e-pub ahead of print).
Hristova EN, Krishnamurthy S, Ro JY et al. Pulmonary epithelioid hemangioendothelioma with prominent signet ring cell features mimicking metastatic adenocarcinoma. Ann Diagn Pathol 2003;7:160–164.
Sheibani K, Battifora H . Signet-ring cell melanoma. A rare morphologic variant of malignant melanoma. Am J Surg Pathol 1988;12:28–34.
LiVolsi VA, Brooks JJ, Soslow R et al. Signet cell melanocytic lesions. Mod Pathol 1992;5:515–520.
Grilliot MA, Goldblum JR, Liu X . Signet-ring cell melanoma of the gastroesophageal junction: a case report and literature review. Arch Pathol Lab Med 2012;136:324–328.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ordóñez, N. Mesothelioma with signet-ring cell features: report of 23 cases. Mod Pathol 26, 370–384 (2013). https://doi.org/10.1038/modpathol.2012.172
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2012.172
Keywords
This article is cited by
-
Epithelioid Hemangioendothelioma: clinicopathologic, immunhistochemical, and molecular genetic analysis of 39 cases
Diagnostic Pathology (2014)
-
The carcinogenic effect of various multi-walled carbon nanotubes (MWCNTs) after intraperitoneal injection in rats
Particle and Fibre Toxicology (2014)
-
Follicular Thyroid Carcinoma with Signet Ring Cell Morphology: Fine-Needle Aspiration Cytology, Histopathology, and Immunohistochemistry
Endocrine Pathology (2013)