Abstract
Mesothelial tumors are classified into benign or preinvasive tumors, and mesotheliomas. The benign or preinvasive group includes adenomatoid tumors, well-differentiated papillary mesothelial tumors, and mesothelioma in situ. Malignant tumors are mesotheliomas and can be localized or diffuse. Histological classification of invasive mesotheliomas into three major subtypes—epithelioid, sarcomatoid, and biphasic is prognostically important. It also plays a significant role in the treatment decisions of patients diagnosed with this deadly disease. Grading and subtyping of epithelioid mesotheliomas have been one of the major changes in the recent WHO classification of pleural tumors. Mesothelioma in situ has emerged as a precisely defined clinico-pathologic entity that for diagnosis requires demonstration of loss of BAP1 or MTAP by immunohistochemistry, or CDKN2A homozygous deletion by FISH. The use of these two biomarkers improves the diagnostic sensitivity of effusion specimens and limited tissue samples and is valuable in establishing the diagnosis of epithelioid mesothelioma. In this review, recent changes in the histologic classification of pleural mesothelioma, importance of ancillary diagnostic studies, and molecular characteristics of mesotheliomas are discussed.
Similar content being viewed by others
Mesothelial tumors are classified into benign or preinvasive tumors, and mesotheliomas1. The benign or preinvasive group includes adenomatoid tumors, well-differentiated papillary mesothelial tumors and mesothelioma in situ. Malignant tumors are mesotheliomas and can be localized or diffuse1.
Malignant tumors in many organs have an in situ phase that can be diagnosed microscopically. In contrast, mesothelioma in situ has long been a controversial topic. The initial reports that described what the authors believed was mesothelioma in situ were all seen in a background of an invasive mesothelioma and probably represented the surface spread of an invasive mesothelioma2,3. The concept of mesothelioma in situ as a clinico-pathologic entity was recently described and for the first time included in the 2021 WHO classification4,5,6. Since morphology is insufficient for unequivocal diagnosis of mesothelioma in situ, demonstration of loss of BAP1 or MTAP by immunohistochemistry (IHC) or CDKN2A homozygous deletion is essential. These markers emerged as specific diagnostic markers of malignancy in mesothelial proliferations and allowed the diagnosis of mesothelioma in fluid specimens and limited tissue samples7,8,9,10,11,12,13,14.
Pleural mesothelioma is a rare tumor associated with poor prognosis. The WHO classifications of pleural mesothelioma traditionally recognized the three major subtypes of epithelioid, biphasic, and sarcomatoid. These major subtypes have an impact on prognosis and treatment of patients diagnosed with this aggressive tumor15. According to the SEER database, the median survival in patients diagnosed with epithelioid, biphasic, and sarcomatoid mesotheliomas of the pleura who underwent surgical treatment was 19, 12, and 4 months, respectively15. Histologic subtype also determines the treatment with surgery being recommended for epithelioid mesothelioma only16,17.
Morphological heterogeneity of epithelioid mesothelioma has been recognized for a long time, but only recently prognostic significance of different architectural patterns has been reported18,19. In addition to architectural patterns, prognostic significance of nuclear grade, mitotic count and necrosis has been recognized20,21,22,23. Both architectural patterns and grading of epithelioid mesothelioma may provide better stratification of the patents in regards to clinical management. In contrast, the criteria for diagnosis of biphasic and sarcomatoid mesotheliomas remain unchanged.
This review will provide an update on newly proposed concepts in the diagnosis of pleural mesothelioma.
Preinvasive mesothelial tumors
Mesothelioma in situ
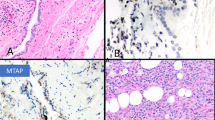
The originally proposed definition of mesothelioma in situ was based on morphology alone, and was described as a single layer of small papillary projections of cytologically atypical mesothelial cells on a pleural surface2,3. These originally reported cases were seen in a background of invasive mesothelioma, and the argument was whether this growth truly represented mesothelioma in situ or surface spread of an underlying invasive mesothelioma. Although it was believed that mesothelioma in situ must exist, the consensus among experts was that mesothelioma in situ cannot be distinguished from reactive/atypical proliferations on morphology alone. Advances in understanding of molecular events responsible for development of malignant mesothelial proliferations particularly BAP1 gene alterations and CDKN2A homozygous deletion that can be identified by clinically validated assays allowed identification of those early malignant lesions8,24,25,26. Churg et al. were first to report the cases of mesothelioma in situ with flat or slightly papillary single layer surface mesothelial proliferation with BAP1 loss and/or CDKN2A homozygous deletion in patients with recurrent non-resolving pleural effusions and without evidence of tumor on imaging or thoracoscopy (Fig. 1)4,5. These changes tend to occur in the setting of heavy asbestos exposure, post radiation, and in patients with familial predisposition. Flat proliferations show no or minimal cytologic atypia, while moderate-to-severe atypia can be seen in small papillary proliferations. Mitoses are typically absent. It is worth emphasizing that morphology is insufficient for diagnosis of mesothelioma in situ, and demonstration of BAP1 loss by immunohistochemistry or CDKN2A homozygous deletion by FISH is required for diagnosis1,4,5. MTAP IHC can be used as a surrogate for CDKN2A FISH assay7,27,28. It is essential that these assays are rigorously validated in order to prevent misdiagnosing mesothelioma in situ. Whole-exome sequencing confirmed that mesothelioma in situ development is associated with BAP1 somatic mutations/deletions, and suggested that BAP1 alterations represent a very early event in the development of a subset of mesotheliomas29. It is currently unknown what other genetic alterations represent an early event in mesothelioma in situ as BAP1 and CDKN2A loss occur in up to 70% of cases. Even though the diagnosis of malignant mesothelial proliferations can be established in fluid specimens, the diagnosis of mesothelioma in situ cannot be made in cytology specimens, and the tissue sample is needed to rule out invasion. The management of patients with mesothelioma in situ should be discussed with multidisciplinary clinical team as there is usually a long latency period before patient present with an invasive mesothelioma, however, after a median follow up of 5 years up to 70% mesotheliomas in situ will progress into invasive mesothelioma5.
(A) A single layer of monotonous flat mesothelial cells (H&E, ×40) (B) BAP1 loss in neoplastic mesothelial cells, while intact in stromal and inflammatory cell (IHC, ×20) (C) MTAP IHC loss in mesothelial cells as a surrogate marker for CDKN2A homozygous deletion (IHC, ×20) (from ref. 5).
Diffuse mesothelioma
Epithelioid mesothelioma
Table 1 summarizes architectural patterns and cytological features of epithelioid mesothelioma. Some cases also show myxoid stromal features. It is important to be aware of morphological heterogeneity of epithelioid mesothelioma in order to perform adequate immunohistochemical workup and to avoid misdiagnosis. Immunohistochemical diagnostic workup of epithelioid mesotheliomas has been well established, and extensively reviewed elsewhere12,30.
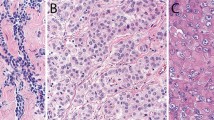
Furthermore, prognostic significance of some architectural patterns has been reported. Two architectural patterns that were considered epithelioid patterns, but show prognosis similar to sarcomatoid and biphasic mesotheliomas, are pleomorphic and transitional18,19,31. These two morphologies are in the 2021 WHO classification reclassified as cytological features (Table 1)1. Recent transcriptome study strongly supported reclassification of transitional features as sarcomatoid31. Transitional features have appearance between epithelioid and sarcomatoid morphology, showing a sheet-like elongated but plump cell with well-defined cell borders (Fig. 2). It is extremely important to recognize this morphology in order to classify mesothelioma as biphasic, if second epithelioid component is present. However, the diagnostic interobserver reproducibility is fair based on H&E alone (wK = 0.40)32. In difficult cases, pathologists may choose to do reticulin stain which may help to distinguish transitional features from an epithelioid subtype. Reticulin stain highlights clusters of cells in epithelioid subtype, while in sarcomatoid and transitional subtypes single cells are highlighted31. Pleomorphic features, as described in epithelioid mesothelioma, can also be seen in sarcomatoid mesotheliomas. In contrast to transitional features, emerging genomic data do not support reclassification of pleomorphic features and the consensus is to classify it based on the most predominant morphology as either sarcomatoid or epithelioid1,33.
A Transitional features show sheet-like growth of the plump, elongated, cohesive cells with well-defined borders. (H&E stain, magnification ×40) (from ref. 34). B Pleomorphic features with large cells with abundant cytoplasm and large highly atypical nuclei (H&E stain, magnification ×40).
One of the major changes in the 2021 WHO classification of epithelioid mesothelioma is grading. Grading has not been recommended for mesotheliomas previously, and this change is based on published studies that demonstrated prognostic significance of various morphological features such as nuclear atypia, mitotic count, and necrosis20,21,22,23. A two-tier system of low and high grade that is applicable to resections and biopsies with epithelioid mesotheliomas has been recommended (Fig. 3 and Table 1)33,34. This grading system is based on combining nuclear grade (nuclear atypia and mitotic count) and presence of necrosis (Table 1). Areas showing the highest grade should be used to assign mesothelioma grade. Currently, no grading of sarcomatoid or biphasic mesotheliomas is recommended. The role of aggressive architectural patterns in grading is uncertain at this point.
Sarcomatoid mesothelioma
Sarcomatoid mesothelioma is the second most common subtype and has been associated with only 4 months survival in patients who underwent surgical treatment15,16. The WHO classification defines it as a proliferation of spindle cells arranged in fascicles or in haphazard patterns invading the adipose tissue and/or lung parenchyma1. Necrosis and atypical mitoses are frequently present. Heterologous elements such as osteosarcoma, rhabdomyosarcoma, or chondrosarcoma can be present in rare cases. Table 1 summarizes variants and cytological features of sarcomatoid mesothelioma. Desmoplastic mesothelioma is diagnostically most challenging as it shows spindle cells with minimal atypia arranged haphazardly in a so-called patternless pattern within a dense hyalinized stroma that resemble pleural hyaline plaque. The presence of obvious sarcomatoid areas is very helpful in establishing the diagnosis, as this variant may easily be interpreted as benign. In difficult cases, ancillary studies, particularly detection of CDKN2A homozygous deletion or loss of MTAP by IHC are very helpful, as >90% of sarcomatoid mesothelioma harbor this alteration7,27,35. In contrast to epithelioid mesotheliomas, BAP1 loss is less frequent in sarcomatoid mesotheliomas, and therefore, less helpful in distinction from benign processes36,37.
Immunohistochemical workup of sarcomatoid mesotheliomas is usually more extensive and different from epithelioid mesotheliomas12,38. It should include, in addition to cytokeratins and mesothelial markers, a panel of mesenchymal markers such as desmin, S-100 protein, myogenin, STAT6, CD34, ERG, CD31, FLI1, and also melanoma markers (SOX10, HMB45, and melan A)12. Carcinoma markers such as claudin 4, MOC31, Ber-EP4, and CEA are not very helpful in the differential diagnosis of sarcomatoid tumors and do not need to be included in the panel, particularly if tissue is limited12. In the differential diagnosis from sarcomatoid carcinomas, organ site and differentiation specific markers such as TTF-1 and p40 may be helpful. D2–40 (podoplanin) has been shown higher sensitivity in comparison to other markers in establishing the diagnosis of sarcomatoid mesothelioma33,39 Recently, GATA3 immunohistochemistry was suggested as a marker for distinguishing sarcomatoid mesothelioma from sarcomatoid lung carcinoma40,41 Strong and diffuse GATA3 expression is observed in mesotheliomas, while sarcomatoid carcinomas are largely negative or show weak and patchy staining. In cases with focal keratin expression, sarcomas are in the differential diagnosis and the workup should include either FISH or PCR-based studies for sarcoma-specific diagnostic gene fusions.
Biphasic mesothelioma
Biphasic mesotheliomas are composed of both epithelioid and sarcomatoid components. At least 10% of each component is required for definitive diagnosis in resection specimens (extended pleural decortication/extrapleural pneumonectomy). The reported prognostic cutoffs for sarcomatoid component range from 50% to 80%42. However, more data are needed before cutoff changes can be made to the WHO definition. The diagnosis of biphasic mesothelioma, regardless of percentages of each component, can be made in small biopsies33,42. The recommendation is to report percentage of each component in the biopsy33. Cytokeratin expression can be helpful in the assessment of the amount of sarcomatoid component32,42. Cytokeratin expression highlights spindle cell morphology and tends to be more intense in malignant than in benign reactive spindle mesothelial proliferations. As mentioned above, in difficult cases where the distinction between malignant and benign mesothelial spindle cell proliferations are challenging, demonstrations of CDKN2A homozygous deletion or in some cases BAP1 IHC can be helpful.
Diagnostic role of cytology and small samples
The diagnosis of mesothelioma on morphology alone in body fluid effusion specimens and small biopsies can be challenging43. Similar to surgical specimens, immunohistochemical workup should be performed to establish mesothelial origin of the proliferation. This practice emphasize the importance of cell block preparations for fluid samples. The 2018 American Society of Clinical Oncology clinical guidelines for the diagnosis of pleural mesothelioma stated that the cytological evaluation of pleural fluid can be an initial screening test for mesothelioma, but it is not a sufficiently sensitive diagnostic test17. As a result, many cases will be subjected to biopsy procedures even though their yield is variable44,45.
Common features of malignancy such as cytologic atypia, mitoses, necrosis, and high cellularity can be seen in benign reactive mesothelial proliferations and are not as helpful in separation from malignant mesothelioma. It has to be kept in mind that BAP1/MTAP IHC and FISH for CDKN2A homozygous deletion are valuable diagnostic tools for diagnosing mesotheliomas in body fluid cytology or limited tissue biopsies and should be strongly considered in the workup of mesothelial proliferations (Fig. 4). Recently, it was demonstrated that the use of those two markers in the diagnostic workup of effusion specimens can establish the diagnosis of mesothelioma earlier even before the fully developed clinical picture8. Traditionally, tissue invasion was required for the diagnosis of mesothelioma, but implementation of testing for BAP1/MTAP and/or CDKN2A eliminates the need for this diagnostic criterion. Also the issue of entrapped mesothelial cells in reactive process that can be overinterpreted as invasion becomes less of an issue if those ancillary studies are informative.
Initial immunohistochemical work-up to confirm mesothelial proliferation should be followed by markers of malignant mesothelial proliferations BAP1 immunohistochemistry and/or FISH for CDKN2A homozygous deletion or MTAP immunohistochemistry as surrogate marker (modified from ref. 30).
Molecular pathology
Testing for predictive biomarkers of response to non-surgical therapies is not recommended at this time17. In contrast to numerous molecular studies in other tumor types such as lung adenocarcinoma, genomic studies in mesothelioma are still relatively limited24,25,46,47,48. Somatic mutation burden in malignant mesothelioma is low, usually <2 non-synonymous mutations per megabase, and with no difference between histologic subtypes24,25. Somatic copy number alterations primarily deletions, most frequently CDKN2A are the most common genetic events24,25. CDKN2A homozygous loss most frequently occurs in sarcomatoid mesotheliomas, followed by biphasic and epithelioid.
The comprehensive genomic analyses demonstrated that most frequently mutated genes are BAP1, NF2, TP53, SETD2, DDX3X, ULK2, RYR2, CFAP45, SETDB1, and DDX5124,25. Somatic mutations in the BAP1 gene located on chromosome 3p21 occur in over 50% of pleural mesotheliomas, mostly epithelioid, and are often associated with concurrent loss of heterozygosity on chromosome 3p2124,26,49. In the TCGA cohort, a subset of mesotheliomas with TP53 and SETDB1 co-mutations associated with genome-wide LOH that affects more than 80% of the genome (“genomic near-haploidization”) was identified mostly in young female patients24. ALK gene rearrangements were reported in peritoneal mesotheliomas occurring in young women and children, but no such reports exist in pleural mesothelioma50,51,52. EWSR1 fusions have been found in rare cases of epithelioid pleural and peritoneal mesotheliomas in younger patients without history of asbestos exposure53,54.
There were several sequencing efforts in the past several years. Bueno et al. reported four cluster groups of mesothelioma based on expression patterns that mostly matched the 2015 WHO histologic classification and correlated with overall survival25. Those clusters included sarcomatoid, epithelioid, biphasic-epithelioid, and biphasic-sarcomatoid. These groups essentially recapitulated epithelial-to-mesenchymal transition. Similarly, the TCGA cohort identified four distinct prognostic groups based on genomic, transcriptomic, and epigenomic analysis24. Blum et al. by combining transcriptome, methylome, and miRNome analysis, demonstrated that pleural mesotheliomas have different proportions of epithelioid and sarcomatoid components (E- score and S-score)46. The same group also showed the link between those scores and the mesothelioma microenvironment.47 The S-score correlated with the presence of T cells, monocytes, fibroblasts, endothelial cells, and high expression of PD-L1. The E-score was associated with infiltration of NK cells, complement pathway and VISTA overexpression. These results are consistent with reports of frequent association of PD-L1 protein expression and sarcomatoid mesotheliomas, poor prognosis, and increased lymphocytic inflammation55,56,57,58,59,60,61.
References
Sauter, J. L., Bueno, R., Dacic, S., Gill, R. R., Husain, A. N. & Kadota K. et al. Diffuse Pleural Mesothelioma. WHO Classification of Tumors Editorial Board. 5th ed. 204–219 (Lyon IARC Press, 2021).
Whitaker, D., Henderson, D. W. & Shilkin, K. B. The concept of mesothelioma in situ: implications for diagnosis and histogenesis. Semin. Diagn. Pathol. 9, 151–61 (1992).
Henderson, D. W., Shilkin, K. B. & Whitaker, D. Reactive mesothelial hyperplasia vs mesothelioma, including mesothelioma in situ: a brief review. Am. J. Clin. Pathol. 110, 397–404 (1998).
Churg, A. et al. Malignant mesothelioma in situ. Histopathology 72, 1033–8 (2018).
Churg, A. et al. Malignant mesothelioma in situ: morphologic features and clinical outcome. Mod. Pathol. 33, 297–302 (2020).
Minami, K. et al. Malignant mesothelioma in situ diagnosed by methylthioadenosine phosphorylase loss and homozygous deletion of CDKN2A: a case report. Virchows Arch. 476, 469–73 (2020).
Berg, K. B., Churg, A. M., Cheung, S. & Dacic, S. Usefulness of methylthioadenosine phosphorylase and BRCA-associated protein 1 immunohistochemistry in the diagnosis of malignant mesothelioma in effusion cytology specimens. Cancer Cytopathol. 128, 126–32 (2020).
Chevrier, M., Monaco, S. E., Jerome, J. A., Galateau-Salle, F., Churg, A. & Dacic, S. Testing for BAP1 loss and CDKN2A/p16 homozygous deletion improves the accurate diagnosis of mesothelial proliferations in effusion cytology. Cancer Cytopathol. 128, 939–47 (2020).
Cigognetti, M. et al. BAP1 (BRCA1-associated protein 1) is a highly specific marker for differentiating mesothelioma from reactive mesothelial proliferations. Mod. Pathol. 28, 1043–57 (2015).
Cozzi, I., Oprescu, F. A., Rullo, E. & Ascoli, V. Loss of BRCA1-associated protein 1 (BAP1) expression is useful in diagnostic cytopathology of malignant mesothelioma in effusions. Diagn. Cytopathol. 46, 9–14 (2018).
Kinoshita, Y. et al. A combination of MTAP and BAP1 immunohistochemistry in pleural effusion cytology for the diagnosis of mesothelioma. Cancer Cytopathol. 126, 54–63 (2018).
Husain, A. N. et al. Guidelines for pathologic diagnosis of malignant mesothelioma 2017 update of the consensus statement from the International Mesothelioma Interest Group. Arch. Pathol. Lab. Med. 142, 89–108 (2018).
Monaco, S., Mehrad, M. & Dacic, S. Recent advances in the diagnosis of malignant mesothelioma: focus on approach in challenging cases and in limited tissue and cytologic samples. Adv. Anat. Pathol. 25, 24–30 (2018).
Wang, L. M. et al. Diagnostic accuracy of BRCA1-associated protein 1 in malignant mesothelioma: a meta-analysis. Oncotarget 8, 68863–72 (2017).
Meyerhoff, R. R. et al. Impact of mesothelioma histologic subtype on outcomes in the surveillance, epidemiology, and end results database. J. Surg. Res. 196, 23–32 (2015).
Mansfield, A. S., Symanowski, J. T. & Peikert, T. Systematic review of response rates of sarcomatoid malignant pleural mesotheliomas in clinical trials. Lung Cancer 86, 133–6 (2014).
Kindler, H. L. et al. Treatment of malignant pleural mesothelioma: american society of clinical oncology clinical practice guideline. J. Clin. Oncol. 36, 1343–73 (2018).
Brcic, L., Vlacic, G., Quehenberger, F. & Kern, I. Reproducibility of malignant pleural mesothelioma histopathologic subtyping. Arch. Pathol. Lab. Med. 142, 747–52 (2018).
Kadota, K. et al. Pleomorphic epithelioid diffuse malignant pleural mesothelioma: a clinicopathological review and conceptual proposal to reclassify as biphasic or sarcomatoid mesothelioma. J. Thorac. Oncol. 6, 896–904 (2011).
Kadota, K. et al. A nuclear grading system is a strong predictor of survival in epitheloid diffuse malignant pleural mesothelioma. Mod. Pathol. 25, 260–71 (2012).
Pelosi, G. et al. Pathologic grading of malignant pleural mesothelioma: an evidence-based proposal. J. Thorac. Oncol. 13, 1750–61 (2018).
Rosen, L. E. et al. Nuclear grade and necrosis predict prognosis in malignant epithelioid pleural mesothelioma: a multi-institutional study. Mod. Pathol. 31, 598–606 (2018).
Zhang, Y. Z. et al. Utility of nuclear grading system in epithelioid malignant pleural mesothelioma in biopsy-heavy setting: an external validation study of 563 cases. Am. J. Surg. Pathol. 44, 347–56 (2020).
Hmeljak, J. et al. Integrative molecular characterization of malignant pleural mesothelioma. Cancer Discov. 8, 1548–65 (2018).
Bueno, R. et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat. Genet. 48, 407–16 (2016).
Carbone, M. et al. BAP1 cancer syndrome: malignant mesothelioma, uveal and cutaneous melanoma, and MBAITs. J. Transl. Med. 10, 179 (2012).
Berg, K. B., Dacic, S., Miller, C., Cheung, S. & Churg, A. Utility of methylthioadenosine phosphorylase compared with BAP1 immunohistochemistry, and CDKN2A and NF2 fluorescence in situ hybridization in separating reactive mesothelial proliferations from epithelioid malignant mesotheliomas. Arch. Pathol. Lab. Med. 142, 1549–53 (2018).
Hida, T. et al. Immunohistochemical detection of MTAP and BAP1 protein loss for mesothelioma diagnosis: comparison with 9p21 FISH and BAP1 immunohistochemistry. Lung Cancer 104, 98–105 (2017).
Dacic, S. et al. Whole exome sequencing reveals BAP1 somatic abnormalities in mesothelioma in situ. Lung Cancer 149, 1–4 (2020).
Beasley, M. B., Galateau-Salle, F. & Dacic, S. Pleural mesothelioma classification update. Virchows Arch. 478, 59–72 (2021).
Galateau Salle, F. et al. Comprehensive molecular and pathologic evaluation of transitional mesothelioma assisted by deep learning approach: a multi-institutional study of the international mesothelioma panel from the MESOPATH reference center. J. Thorac. Oncol. 15, 1037–53 (2020).
Dacic, S. et al. Interobserver variation in the assessment of the sarcomatoid and transitional components in biphasic mesotheliomas. Mod. Pathol. 33, 255–62 (2020).
Nicholson, A. G. et al. EURACAN/IASLC proposals for updating the histologic classification of pleural mesothelioma: towards a more multidisciplinary approach. J. Thorac. Oncol. 15, 29–49 (2020).
Schulte, J. J., Chapel, D. B., Attanoos, R., Brcic, L., Burn, J. & Butnor K. J. et al. Comparison of nuclear grade, necrosis, and histologic subtype between biopsy and resection in pleural malignant mesothelioma. Am. J. Clin. Pathol. https://doi.org/10.1093/ajcp/aqab054 (2021).
Chiosea, S. et al. Diagnostic importance of 9p21 homozygous deletion in malignant mesotheliomas. Mod. Pathol. 21, 742–7 (2008).
De Rienzo, A. et al. Large-scale analysis of BAP1 expression reveals novel associations with clinical and molecular features of malignant pleural mesothelioma. J. Pathol. 253, 68–79 (2021).
Sheffield, B. S. et al. BAP1 immunohistochemistry and p16 FISH to separate benign from malignant mesothelial proliferations. Am. J. Surg. Pathol. 39, 977–82 (2015).
Marchevsky, A. M. et al. The differential diagnosis between pleural sarcomatoid mesothelioma and spindle cell/pleomorphic (sarcomatoid) carcinomas of the lung: evidence-based guidelines from the International Mesothelioma Panel and the MESOPATH National Reference Center. Hum. Pathol. 67, 160–8 (2017).
Churg, A. et al. Highlights of the 14th international mesothelioma interest group meeting: Pathologic separation of benign from malignant mesothelial proliferations and histologic/molecular analysis of malignant mesothelioma subtypes. Lung Cancer 124, 95–101 (2018).
Prabhakaran, S., Hocking, A., Kim, C., Hussey, M. & Klebe, S. The potential utility of GATA binding protein 3 for diagnosis of malignant pleural mesotheliomas. Hum. Pathol. 105, 1–8 (2020).
Berg, K. B. & Churg, A. GATA3 immunohistochemistry for distinguishing sarcomatoid and desmoplastic mesothelioma from sarcomatoid carcinoma of the lung. Am. J. Surg. Pathol. 41, 1221–5 (2017).
Galateau Salle, F. et al. New INsights on Diagnostic Reproducibility of Biphasic Mesotheliomas: a multi-institutional evaluation by the international mesothelioma panel from the MESOPATH reference center. J. Thorac. Oncol. 13, 1189–203 (2018).
Henderson, D. W., Reid, G., Kao, S. C., van Zandwijk, N. & Klebe, S. Challenges and controversies in the diagnosis of mesothelioma: Part 1. Cytology-only diagnosis, biopsies, immunohistochemistry, discrimination between mesothelioma and reactive mesothelial hyperplasia, and biomarkers. J. Clin. Pathol. 66, 847–53 (2013).
Maskell, N. A., Gleeson, F. V. & Davies, R. J. Standard pleural biopsy versus CT-guided cutting-needle biopsy for diagnosis of malignant disease in pleural effusions: a randomised controlled trial. Lancet. 361, 1326–30 (2003).
Metintas, M. et al. CT scan-guided abrams’ needle pleural biopsy versus ultrasound-assisted cutting needle pleural biopsy for diagnosis in patients with pleural effusion: a randomized, controlled trial. Respiration 91, 156–63 (2016).
Blum, Y. et al. Dissecting heterogeneity in malignant pleural mesothelioma through histo-molecular gradients for clinical applications. Nat. Commun. 10, 1333 (2019).
Alcala, N. et al. Redefining malignant pleural mesothelioma types as a continuum uncovers immune-vascular interactions. EBioMedicine 48, 191–202 (2019).
Quetel, L. et al. Genetic alterations of malignant pleural mesothelioma: association with tumor heterogeneity and overall survival. Mol. Oncol. 14, 1207–23 (2020).
Panou, V. et al. Frequency of germline mutations in cancer susceptibility genes in malignant mesothelioma. J. Clin. Oncol. 36, 2863–71 (2018).
Argani, P. et al. Pediatric mesothelioma with ALK fusions: a molecular and pathologic study of 5 cases. Am. J. Surg. Pathol. 45, 653–61 (2021).
Hung, Y. P. et al. Identification of ALK rearrangements in malignant peritoneal mesothelioma. JAMA Oncol. 4, 235–8 (2018).
Mian, I. et al. Anaplastic lymphoma kinase gene rearrangement in children and young adults with mesothelioma. J. Thorac. Oncol. 15, 457–61 (2020).
Desmeules, P. et al. A subset of malignant mesotheliomas in young adults are associated with recurrent EWSR1/FUS-ATF1 fusions. Am. J. Surg. Pathol. 41, 980–8 (2017).
Panagopoulos, I. et al. RNA sequencing identifies fusion of the EWSR1 and YY1 genes in mesothelioma with t(14;22)(q32;q12). Genes Chromosomes Cancer 52, 733–40 (2013).
Wadowski, B., Bueno, R., & De Rienzo, A. Immune microenvironment and genetics in malignant pleural mesothelioma. Front. Oncol. 11, 684025 (2021).
Napoli, F., Listi, A., Zambelli, V., Witel, G., Bironzo, P. & Papotti, M. et al. Pathological characterization of tumor immune microenvironment (TIME) in malignant pleural mesothelioma. Cancers 13 (2021).
Brcic, L. et al. Prognostic impact of PD-1 and PD-L1 expression in malignant pleural mesothelioma: an international multicenter study. Transl. Lung Cancer Res. 10, 1594–607 (2021).
Fusco, N. et al. Characterization of the immune microenvironment in malignant pleural mesothelioma reveals prognostic subgroups of patients. Lung Cancer 150, 53–61 (2020).
Brosseau, S. et al. Shorter survival in malignant pleural mesothelioma patients with high PD-L1 expression associated with sarcomatoid or biphasic histology subtype: a series of 214 cases from the Bio-MAPS cohort. Clin. Lung Cancer 20, e564–e575 (2019).
Sobhani, N. et al. Tumour infiltrating lymphocytes and PD-L1 expression as potential predictors of outcome in patients with malignant pleural mesothelioma. Mol. Biol. Rep. 46, 2713–20 (2019).
Combaz-Lair, C. et al. Immune biomarkers PD-1/PD-L1 and TLR3 in malignant pleural mesotheliomas. Hum. Pathol. 52, 9–18 (2016).
Author information
Authors and Affiliations
Contributions
S.D. performed writing and review of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dacic, S. Pleural mesothelioma classification—update and challenges. Mod Pathol 35 (Suppl 1), 51–56 (2022). https://doi.org/10.1038/s41379-021-00895-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-021-00895-7
This article is cited by
-
Adenomatoid mesothelioma arising from the diaphragm: a case report and review of the literature
Journal of Medical Case Reports (2022)