Abstract
BRAFV600E mutation has emerged as a marker of aggressive behavior in papillary thyroid carcinoma but its significance in microcarcinoma is not entirely clear. One-hundred and twenty-nine papillary thyroid microcarcinomas were tested for BRAFV600E mutation by single-strand conformation polymorphism, and their clinicopathologic features (age, sex, tumor size, multifocality, nodal metastases, histologic subtype, tumor cell morphology, architecture, tumor-associated stromal reaction, tumor interface to non-neoplastic thyroid (well circumscribed vs infiltrative), extrathyroidal extension, lymphovascular invasion, intratumoral multinucleated giant cells, and adjacent non-neoplastic thyroid pathology) were examined. Compared with tumors without the mutation (39/129, 30%), the mutated microcarcinomas (90/129, 70%) showed significantly higher prevalence of infiltrative tumor borders (78/90 vs 23/39, P=0.001), tumor-associated stromal desmoplasia/fibrosis and/or sclerosis (80/90 vs 25/39, P=0.002), classic nuclear features of papillary thyroid carcinoma (90/90 vs 35/39, P=0.008) and cystic change (43/90 vs 11/39, P=0.05). BRAFV600E mutation was more frequent in classic (75%), tall cell (91%), and other variants (>70%) than in follicular variant (21%) of papillary thyroid microcarcinoma. Tumors without the mutation were significantly more likely to be solid, well circumscribed, and lacked desmoplasia/fibrosis or sclerosis. However, on multivariate analysis, only the follicular variant of papillary microcarcinoma was significantly associated with the absence of mutation (odds ratio (95% confidence interval): 0.09 (0.01–0.54)). Lymph node metastases (n=24) were more frequent in microcarcinomas with mutation than without (21/24 vs 3/24, P=0.02). All patients with lateral cervical node metastasis (n=9), and all but one tumor with extrathyroidal extension (n=17/18) showed BRAFV600E mutation. No significant differences were noted in age, sex, tumor size, multifocality, lymphovascular invasion, psammoma bodies, stromal calcification, intratumoral multinucleated osteoclastic-type giant cells, and lymphocytic infiltration between the two groups of tumors. BRAFV600E mutation is an early event in thyroid carcinogenesis, and is associated with distinctive morphology and aggressive features even in papillary thyroid microcarcinomas.
Similar content being viewed by others
Main
Papillary thyroid microcarcinoma defined by the World Health Organization as a papillary thyroid carcinoma of < or equal to 1 cm in size is increasingly being recognized due to the widespread use of thyroid ultrasonography, and ultrasound-guided fine needle aspiration cytology.1 A 2.4-fold increase in the incidence of thyroid cancer was noted from 1973 to 2002 that was almost entirely due to papillary carcinoma, approximately half of which were microcarcinomas.2 In patients older than 45 years, the most common papillary thyroid carcinoma is microcarcinoma.3 Fortunately, tumor-related mortality has remained very low, and reported to be 0.5% for microcarcinoma.4 Despite this, the optimal management of microcarcinoma remains a subject of ongoing debate. A subset of microcarcinoma is associated with local recurrence and nodal metastasis.4, 5 The identification of this aggressive subset is critical to better triaging of the patients with regard to the need for additional therapy.6
Clinicopathological factors, eg, age more than 45 years, tumor size greater than 5 mm, male sex, multifocality, lymph nodes metastasis, and extrathyroidal extension have been reported to predict poor prognosis.4, 7, 8 In recent years, a T1799A point mutation in the v-raf murine sarcoma viral oncogene homolog B1 (BRAF) resulting in a valine-to-glutamic acid switch at codon 600 (V600E) has emerged as a marker of aggressive behavior in papillary thyroid carcinoma.9 A few studies have suggested that this mutation may predict aggressive behavior in papillary microcarcinoma too.10, 11, 12 Previously we demonstrated that papillary thyroid carcinoma with BRAFV600E mutation was morphologically distinctive.13 We now investigate the incidence of BRAFV600E mutation in papillary microcarcinoma, and ask if the mutated tumors are morphologically distinctive and associated with aggressive features despite their small size.
Materials and methods
All consecutive cases of papillary thyroid microcarcinomas diagnosed at the Department of Pathology at Yale New Haven Hospital from January 2010 to November 2011 were reviewed, and microcarcinomas that underwent testing for BRAFV600E mutation prospectively at the time of initial diagnosis were included in the study (n=129). Microcarcinomas that could not be tested for the mutation were rejected (n=117). The reasons for not testing/exclusion included: inadequate tumor tissue, very small tumors (<1 mm), synchronous larger tumors that were preferred for mutational analysis, and reasons to suspect that the microcarcinoma was not sampled on fine needle aspiration, such as needle tract associated with a thyroid nodule in a different location away from the microcarcinoma. Thus, 129 tumors in 124 patients (total thyroidectomy=110, lobectomies=14) were included in this study. All thyroids were serially sectioned from superior to inferior at 3–4 mm intervals and the cut-surfaces were carefully examined. Sixty-four thyroids were entirely submitted for microscopic examination while representative sections were submitted in the remaining cases. Clinicopathologic information gathered from pathology reports included age, gender, tumor size, number of tumor foci, non-neoplastic thyroid pathology, laterality, lymph node involvement, and surgical procedure. Hematoxylin and eosin stained slides were reviewed by two pathologists (RV and MLP) blinded to the mutational status of the tumors. The following histological features were evaluated: tumor interface with non-neoplastic thyroid (well circumscribed or encapsulated vs infiltrative), extrathyroidal extension, characteristics of tumor cells including tall cells, and polygonal cells with moderate amount of homogeneous eosinophilic cytoplasm (‘plump pink cells’), nuclear features, tumor-associated stromal reaction including desmoplasia, fibrosis and sclerosis, stromal calcification, lymphocytic infiltrate, psammoma bodies, lymphovascular invasion, cystic change, back-to-back arrangement, intratumoral multinucleated giant cells, and any additional pathology in the non-neoplastic thyroid. An attempt was made to determine the histologic variant using the World Health Organization criteria (2004) for larger papillary thyroid carcinoma and included: classic, follicular, tall cell, subcapsular sclerosing, occult sclerosing, Warthin-like, or oncocytic variant (Figures 1 and 2).1 The subcapsular sclerosing variant was defined as a predominantly non-cystic microcarcinoma with (1) a peripheral subcapsular location, (2) abutting and involving the thyroid capsule along at least 20% of the tumor’s circumference, (3) a sclerosing pattern of infiltration, usually with a central stellate scar, and (4) with no or few papillae (Figure 2a). Occult sclerosing variant was differentiated from the subcapsular sclerosing variant by its more central intraparenchymal location (Figures 2c and d). The tumor was determined to be unclassifiable when it did not fit into any of above categories, and when too small to be classified. Desmoplasia, fibrosis, and sclerosis were defined on a spectrum with a tissue culture like plump fibroblastic proliferation in loose myxoid stroma (desmoplasia) at one end, and a paucicellular thick collagenized stroma (sclerosis) at the other end of the spectrum (Figure 2d). Tall cells were defined as tumor cells with height at least twice as tall as wide occupying >50% of the tumor (Figures 1d and e).14 Polygonal tumor cells with moderate to abundant homogeneous eosinophilic cytoplasm and not meeting the ‘tallness’ criteria (plump pink cells) were noted (Figure 2b). The tumor cell nuclei were assessed for six characteristic features of papillary thyroid carcinoma that included (1) nuclear enlargement, (2) overlapping, (3) irregular nuclear membrane, (4) grooves, (5) chromatin clearing, and (6) intranuclear pseudoinclusions. The nuclear features were considered well developed or classic if at least five of six features (≥5) were present and subtle when fewer features (≤4) were present (Figures 1a and c). In the presence of multifocality, the histological features of only the tumor subjected to mutational analysis were included in the data analysis. In five cases, mutational testing was done on two tumor foci in each patient: both tumors were positive for BRAFV600E mutation in three patients and negative in two patients. In these cases, the histological features were separately recorded for each of the tumors.
Papillary thyroid microcarcinoma. (a) Classic variant (7 mm) in a 35-year-old woman showing papillae with central fibrovascular cores. Tumor cells have well-developed nuclear features including nuclear enlargement, overlapping, chromatin clearing, nuclear grooves and irregular nuclear membranes line. (b, c) Follicular variant (10 mm) in an 80-year-old man: Well-circumscribed thinly encapsulated tumor with microfollicular architecture. Higher magnification (c) shows subtle nuclear features of papillary carcinoma. (d, e) Tall cell variant (9 mm) in a 34-year-old woman: the tumor shows trabecular architecture and back-to-back glands with slit-like spaces. Higher magnification (e) shows tumor cells with moderately abundant pink cytoplasm that are at least twice as tall as wide. A mutlinucleated giant cell is also present.
(a) Subcaspular sclerosing variant of papillary thyroid microcarcinoma (9 mm) in a 23-year-old woman. The tumor extends into extrathyroidal soft tissue and shows fibrosis and sclerosis. (b) Microcarcinoma (7 mm) in a 33-year-old woman showing desmoplastic stroma and plump pink cells at the peripheral infiltrative front of the tumor. Non-neoplastic thyroid follicles are appreciated on the right side. (c) Occult sclerosing variant (1 mm) in a 57-year-old woman: The tumor is intraparenchymal. Higher magnification (d) shows tumor-associated sclerosis and characteristic nuclear features of papillary thyroid carcinoma.
BRAFV600E Mutational Analysis
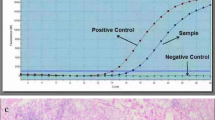
BRAFV600E mutational analysis was performed on fine needle aspiration samples from thyroid nodules with a cytologic diagnosis of indeterminate (atypia of undetermined significance), suspicious or positive for papillary thyroid carcinoma (Bethesda system, 2008).15 After making direct smears for cytologic evaluation, the needle was rinsed in Cytorich Red solution (Thermo-Fisher Scientific, Kalamazoo, MI, USA) for ThinPrep (Hologic, Boxborough, MA, USA) and molecular analysis for BRAFV600E mutation as described previously.16 Tumors that could not be tested on fine needle aspiration were tested on formalin-fixed paraffin-embedded tissues from the thyroid resections after being marked on the hematoxylin and eosin stained slide under the microscope by a board certified pathologist to ensure tumor representation. Five to 10 unstained sections (2–5 μm thick) from the tumor were deparaffinized and macro-dissected followed by DNA extraction using Qiagen tissue kit according to the manufacturer’s protocol (Qiagen, Chatsworth, CA, USA). Five to 20 ng of extracted DNA was amplified using 0.2 μM PCR primers flanking the region of T1799A mutation of BRAF ( flank primer: 5′-CTCTTCATAATGCTTGCTCTGATAGG-3′ and flank primer: 5′-TAGTAACTCAGCAGCATCTCAGG-3′) in a 50 μl PCR reaction solution containing 1 × PCR buffer, 0.1 mM dNTP, 1.5 mM MgCl2 and 2.5 units of AmpliTaq Gold DNA polymerase. PCR started with initial denaturation at 95 °C for 8 min, followed by 35 cycles of denaturation at 94 °C for 1 min, annealing at 55 °C for 1 min and synthesis at 72 °C for 2 min, and finished by a final extension at 72 °C for 10 min (ABI Veriti Thermal Cycler, Applied Biosystems, Foster City, CA, USA). The 250 base pair amplification product was analyzed by single-strand conformation polymorphism using 4 μl of the PCR product on MDE non-denaturing gel. Electrophoresis was carried out on ice for 2 h and 45 min at 325 volts. The gel was then stained with SYBR Gold (Molecular Probes) 1:10 000 in TE added for 20 min and imaged by BioRad GelDoc UV System (BioRad, Hercules, CA, USA). BRAFV600E mutation was determined by comparing the banding pattern of the case with a positive control.
Statistical Analysis
In papillary thyroid microcarcinomas with and without BRAFV600E mutation, the association between categorical and continuous variables was evaluated using the two-tailed Fisher’s exact test and Student’s t-test, respectively (GraphPad InStat 3.1, GraphPad, San Diego, CA, USA). A multivariable unconditional logistic regression model was constructed adjusting for parameters significant on univariate analysis (SAS v9.1.3, SAS Institute, Cary, NC, USA). Model fit was assessed by the area under the receiver–operating curve. For all analysis, α was set at ≤0.05.
Results
A total of 473 patients were diagnosed with papillary thyroid carcinoma during the study period, of which 246 patients had microcarcinoma (52%). Of these, 124 patients with 129 microcarcinomas met the inclusion criteria. Twenty-three microcarcinomas (18%) were incidental as they were not detected by fine needle aspiration or imaging studies preoperatively, and the thyroidectomy was performed for other reasons, eg, goiter (n=17), Graves’ disease (n=3), Hashimoto's thyroiditis (n=1), and Hurthle cells neoplasm (n=2, one each in ipsilateral and contralateral lobe). Seventy-eight microcarcinomas were tested for BRAFV600E mutation upon thyroidectomy, ie, formalin-fixed paraffin-embedded tumor tissues, whereas in 51 tumors the mutational analysis was performed on the fine needle aspirate needle rinses. The detection rate for the mutation was slightly higher in the former (57/78, 73% positive) than the latter samples (33/51, 65% positive).
Table 1 summarizes the patient demographics of 90 BRAFV600E mutation-positive (70%) and 39 mutation-negative (30%) microcarcinomas. The majority (83%) of the patients was women and 63% of patients were older than 45 years. Multifocality was seen in 47% of all tumors. No significant differences were noted in age, sex, tumor size, location, and multifocality between the mutated and non-mutated microcarcinomas.
Lymph nodes were available for histologic evaluation in 87 of 124 (70%) patients (Table 2). The mean number of central lymph nodes examined were similar in mutated and non-mutated tumors (five lymph nodes), but when combined with lateral cervical node dissection, the mean number increased to 10 lymph nodes in the mutated group. Lateral cervical lymph node dissection was undertaken only after confirmation of metastasis by ultrasound-guided fine needle aspiration, and was exclusive to mutation-positive microcarcinomas. BRAFV600E mutational analysis was available in 40% of tumors (n=51) preoperatively on fine needle aspiration. However, the presence of mutation did not apparently influence the decision to dissect lymph nodes (53% node dissection in mutated vs 68% in non-mutated tumors).
Table 3 shows histopathologic features of BRAFV600E mutation-positive and negative papillary thyroid microcarcinomas. Twenty-eight microcarcinomas (22%) were well circumscribed including 18 completely encapsulated tumors and the remaining 101 microcarcinomas (78%) displayed infiltrative tumor borders (Figures 1 and 2). Significantly, 57% of the circumscribed tumors (16 of 28) were BRAFV600E mutation negative in contrast to 23% of infiltrative (23 of 101) microcarcinoma (P=0.001). Cystic change but not back-to-back arrangement of tumor cells was significantly associated with the mutation, as noted in nearly 80% (43 of 54) of microcarcinoma with cystic change. Tumor-associated stromal reaction, eg, desmoplasia, fibrosis and/or sclerosis (Figures 1 and 2) was significantly more frequent in mutation-positive tumors (P=0.002), but stromal calcification, psammoma bodies, and lymphocytic infiltrate were not. Classic nuclear features of papillary thyroid carcinoma were significantly associated with the mutation. Eighteen microcarcinomas showed minimal microscopic extrathyroidal extension and all but one were positive for BRAFV600E mutation (Figure 2a). Mutated tumors were more frequently associated with tumor cells with moderate amount of homogeneous eosinophilic cytoplasm (plump pink cells), especially at the infiltrating front (Figure 2b), intratumoral osteoclast-like multinucleated giant cells (41 of 52, 79%; Figure 1e) and lymphovascular invasion (14 of 17, 82%) but these findings did not reach statistical significance.
Table 4 shows histologic subtypes of the tumors. BRAFV600E mutation was significantly more common (>70%) in classic, tall cell, subcapsular sclerosing, and occult sclerosing variants of papillary thyroid microcarcinoma as compared with the follicular (21%) variant (P<0.05).
On multivariate analysis only follicular variant of papillary microcarcinoma remained significantly associated with the absence of BRAFV600E mutation (odds ratio (95% confidence interval): 0.09 (0.01–0.54)). No other histologic features were found to be significant on multivariate analysis.
Discussion
Previously we showed that BRAFV600E mutation-positive and negative papillary thyroid carcinomas have distinctive morphology.13 Now we extend our investigation to microcarcinomas (tumor size ≤1 cm), and show that even microcarcinomas have a high prevalence of BRAFV600E mutation, and tumors with the mutation are significantly associated with lymph node metastasis, in particular lateral cervical lymph node metastasis, extrathyroidal extension, infiltrating tumor borders, tumor-associated fibrosis and desmoplasia, cystic change, and classic nuclear features of PTC.
We report a 70% incidence of BRAFV600E mutation in papillary thyroid microcarcinoma, similar to larger papillary thyroid carcinoma.13, 17 Others have reported BRAFV600E mutation in <50% of microcarcinoma.9, 10, 11, 18 Interestingly, a recent study from Korea reported BRAFV600E mutation in 65.6% in papillary thyroid microcarcinoma similar to the current study, but the authors attributed the higher prevalence to population differences.19 In our study as in the Korean study, the microcarcinomas were marked on the hematoxylin and eosin stained glass slide by a pathologist, and macro-dissected for the extraction of tumor DNA. Tumor sampling in surgical pathology material was improved by extracting DNA from 5 to 10 unstained sections depending upon tumor size, whereas on fine needle aspiration, tumor sampling may be limited by one pass or by needle rinse. A validation of single-strand conformation polymorphism for the detection of BRAFV600E in surgical pathology material against direct PCR-Sanger sequencing in our laboratory also showed 76% incidence of the mutation in papillary thyroid carcinoma and confirmed the superior sensitivity and specificity of the former technique over the latter.20 In another study from Italy, Marchetti et al21 reported a prevalence of 74% BRAFV600E mutations in 85 papillary thyroid microcarcinomas. The authors (all cytopathologists) identified tumor cells on stained cytology smears before scraping them off for DNA extraction, and used polymerase chain reaction with direct sequencing for BRAFV600E. Thus, the higher prevalence may be due to technical differences in mutation testing methods, specifically technical steps that ensure tumor representation. In our experience, the involvement of well-trained pathologists has been critical in avoiding false negative results and in improving detection rates of molecular events.
Lymph node metastasis and extrathyroidal extension are predictors of persistent/recurrent disease.6 In papillary thyroid microcarcinoma, nodal metastases have been reported in the range of 6–66.4% cases.4, 5, 7, 19, 22, 23, 24 In the present study, node dissection was performed in 70% (n=87) of the patients. Of these, 28% of patients had metastatic lymph nodes, and 88% of patients with nodal metastasis had microcarcinomas positive for the mutation. Lymph node metastasis was significantly more frequent in BRAFV600E-mutated microcarcinomas, and more importantly, metastases to lateral cervical lymph nodes were exclusively seen in mutated microcarcinomas. However, on multivariate analysis, this association was not significant suggesting that there may be other molecular events in the natural history of microcarcinomas.
Extrathyroidal extension has been reported in 0.02–52.2% in papillary thyroid microcarcinomas.5, 10, 12, 19, 25 We found microscopic extrathyroidal extension in 14% of the microcarcinomas and all but one harbored the mutation. Our study shows the incidence of extrathyroidal extension in relatively less proportion of cases compared with some of the studies in the literature. In our institution, we define extrathyroidal extension conservatively as the thyroid capsule is ill defined and incomplete. Moreover, benign thyroid follicles can be frequently seen outside of the capsule separate from the thyroid parenchyma. In addition, intrathyroidal fat and skeletal muscle can be seen within normal thyroids, further complicating the assessment of extrathyroidal extension.
Lupi et al9 found the absence of the tumor capsule to be the only histological parameter significantly associated with BRAFV600E mutation. We noted that BRAFV600E-mutated tumors typically had infiltrative borders and lacked an intact capsule. We also found that mutated microcarcinomas were significantly more likely to have tumor-associated stromal changes (fibrosis, sclerosis or desmoplasia). Koperek et al26 reported tumor-associated desmoplasia in 74% of papillary thyroid microcarcinomas, and it was significantly associated with lymph node metastasis and tumor diameter. BRAFV600E has been implicated in extracellular matrix remodeling, invasiveness, and desmoplasia.27 The stromal alterations are considered to be a critical step toward tumor progression.
We assigned a histologic subtype to the papillary thyroid microcarcinomas based upon the criteria used for larger papillary thyroid carcinomas. A high prevalence of BRAFV600E mutation was noted in certain histologic subtypes, ie, classic, tall cell, subcapsular and occult sclerosing variants in contrast to the follicular variant of papillary thyroid microcarcinoma. We first noticed this trend in our previous study on BRAFV600E mutation-positive larger tumors.13 Similar to larger papillary thyroid carcinomas, the prevalence of the mutation in tall cell microcarcinomas was >90%, and in classic variant was >70%, whereas the follicular variant consistently showed low prevalence or the absence of the mutation.28, 29 The subcapsular/superficial location of papillary thyroid microcarcinoma is believed to be a significant risk factor for aggressive behavior.30 Therefore, we studied them separately from the classic and the intraparenchymal occult sclerosing types of microcarcinomas but found no significant difference in the prevalence of BRAFV600E mutation. The small size of papillary thyroid microcarcinomas makes histologic subtyping difficult or unclassifiable, and possibly poorly reproducible among pathologists. Despite these limitations, we show that papillary thyroid microcarcinomas have distinctive morphology that correlates significantly with BRAFV600E mutation. Indeed, upon multivariate analysis, only the follicular variant of papillary thyroid microcarcinoma was significantly associated with the absence of BRAFV600E mutation. We conclude that most papillary thyroid microcarcinoma can be and should be histologically subtyped.
Interestingly, mutated microcarcinomas tended to show classic nuclear features of papillary thyroid carcinoma, whereas tumors with subtle nuclear features were mutation negative. Another interesting morphologic feature was the presence of moderate to abundant eosinophilic cytoplasm in tumor cells that were polygonal or somewhat tall but did not quite meet the criteria for tall cell variant (>50% of tumor cells at least twice as tall as wide). For lack of a better terminology, we documented these tumor cells as ‘plump pink cells’. ‘Plump pink cells’ were more common in BRAFV600E-mutated tumors, especially at the infiltrating edge of the tumor, within extrathyroidal extension, in lymph node deposits and in intravascular tumor emboli. No significant association with age, gender, multifocality, tumor size, and other histological features was found with BRAFV600E mutation.
The current study is limited by the lack of clinical follow-up including recurrence, persistence, and progression. Despite this limitation, we show that BRAFV600E-mutated papillary thyroid microcarcinomas demonstrate aggressive features at presentation. Niemeier et al30 have suggested a combined molecular-pathologic scoring system for risk stratification of papillary thyroid microcarcinoma that included: BRAFV600E mutation, superficial location, fibrosis, and multifocality/intraglandular tumor spread but their study too was limited by the absence of follow-up. We found the first three features to be generally present together in mutated microcarcinoma. Further studies with long-term follow-up are needed in the risk stratification of microcarcinomas with BRAFV600E mutation.
In conclusion, our findings suggest that BRAFV600E mutation is an early and phenotypically defining molecular event in papillary thyroid carcinoma. The mutation is associated with features predictive of a high risk of local recurrence, eg, extrathyroidal extension and nodal, specifically lateral cervical lymph node metastasis, even in microcarcinomas. Despite their small size, the phenotype of microcarcinomas with regard to the BRAFV600E mutation is distinctive, and the incidence of mutation remains similar to larger papillary thyroid carcinoma.
References
DeLellis RA, Lloyd RV, Heitz PU, Eng C (eds). World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Endocrine Organs. IARC Press: Lyon, 2004, pp 57–66.
Davies L, Welch HG . Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 2006;295:2164–2167.
Hughes DT, Haymart MR, Miller BS, et al. The most commonly occurring papillary thyroid cancer in the United States is now a microcarcinoma in a patient older than 45 years. Thyroid 2011;21:231–236.
Yu XM, Wan Y, Sippel RS, et al. Should all papillary thyroid microcarcinomas be aggressively treated? An analysis of 18,445 cases. Ann Surg 2011;254:653–660.
Pelizzo MR, Boschin IM, Toniato A, et al. Natural history, diagnosis, treatment and outcome of papillary thyroid microcarcinoma (PTMC): a mono-institutional 12-year experience. Nucl Med Commun 2004;25:547–552.
Xing M . BRAF mutation in papillary thyroid microcarcinoma: the promise of better risk management. Ann Surg Oncol 2009;16:801–803.
Page C, Biet A, Boute P, et al. 'Aggressive papillary’ thyroid microcarcinoma. Eur Arch Otorhinolaryngol 2009;266:1959–1963.
Neuhold N, Schultheis A, Hermann M, et al. Incidental papillary microcarcinoma of the thyroid—further evidence of a very low malignant potential: a retrospective clinicopathological study with up to 30 years of follow-up. Ann Surg Oncol 2011;18:3430–3436.
Lupi C, Giannini R, Ugolini C, et al. Association of BRAF V600E mutation with poor clinicopathological outcomes in 500 consecutive cases of papillary thyroid carcinoma. J Clin Endocrinol Metab 2007;92:4085–4090.
Lee X, Gao M, Ji Y, et al. Analysis of differential BRAF(V600E) mutational status in high aggressive papillary thyroid microcarcinoma. Ann Surg Oncol 2009;16:240–245.
Kwak JY, Kim EK, Chung WY, et al. Association of BRAFV600E mutation with poor clinical prognostic factors and US features in Korean patients with papillary thyroid microcarcinoma. Radiology 2009;253:854–860.
Lin KL, Wang OC, Zhang XH, et al. The BRAF mutation is predictive of aggressive clinicopathological characteristics in papillary thyroid microcarcinoma. Ann Surg Oncol 2010;17:3294–3300.
Finkelstein A, Levy GH, Hui P, et al. Papillary thyroid carcinomas with and without BRAF V600E mutations are morphologically distinct. Histopathology 2012;60:1052–1059.
Ghossein R, Livolsi VA . Papillary thyroid carcinoma tall cell variant. Thyroid 2008;18:1179–1181.
Baloch ZW, Cibas ES, Clark DP, et al. The National Cancer Institute Thyroid fine needle aspiration state of the science conference: a summation. Cytojournal 2008;5:6.
Adeniran AJ, Theoharis C, Hui P, et al. Reflex BRAF testing in thyroid fine-needle aspiration biopsy with equivocal and positive interpretation: a prospective study. Thyroid 2011;21:717–723.
Cohen Y, Xing M, Mambo E, et al. BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst 2003;95:625–627.
Ugolini C, Giannini R, Lupi C, et al. Presence of BRAF V600E in very early stages of papillary thyroid carcinoma. Thyroid 2007;17:381–388.
Park YJ, Kim YA, Lee YJ, et al. Papillary microcarcinoma in comparison with larger papillary thyroid carcinoma in BRAF(V600E) mutation, clinicopathological features, and immunohistochemical findings. Head Neck 2010;32:38–45.
Ziai J, Hui P . BRAF mutation testing in clinical practice. Expert Rev Mol Diagn 2012;12:127–138.
Marchetti I, Iervasi G, Mazzanti CM, et al. Detection of the BRAF(V600E) mutation in fine needle aspiration cytology of thyroid papillary microcarcinoma cells selected by manual macrodissection: an easy tool to improve the preoperative diagnosis. Thyroid 2012;22:292–298.
Wada N, Duh QY, Sugino K, et al. Lymph node metastasis from 259 papillary thyroid microcarcinomas: frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann Surg 2003;237:399–407.
Pelizzo MR, Boschin IM, Toniato A, et al. Papillary thyroid microcarcinoma (PTMC): prognostic factors, management and outcome in 403 patients. Eur J Surg Oncol 2006;32:1144–1148.
So YK, Son YI, Hong SD, et al. Subclinical lymph node metastasis in papillary thyroid microcarcinoma: a study of 551 resections. Surgery 2010;148:526–531.
Kwak JY, Kim EK, Youk JH, et al. Extrathyroid extension of well-differentiated papillary thyroid microcarcinoma on US. Thyroid 2008;18:609–614.
Koperek O, Asari R, Niederle B, et al. Desmoplastic stromal reaction in papillary thyroid microcarcinoma. Histopathology 2011;58:919–924.
Mesa C, Mirza M, Mitsutake N, et al. Conditional activation of RET/PTC3 and BRAFV600E in thyroid cells is associated with gene expression profiles that predict a preferential role of BRAF in extracellular matrix remodeling. Cancer Res 2006;66:6521–6529.
Nikiforova MN, Kimura ET, Gandhi M, et al. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab 2003;88:5399–5404.
Rivera M, Ricarte-Filho J, Tuttle RM, et al. Molecular, morphologic, and outcome analysis of thyroid carcinomas according to degree of extrathyroid extension. Thyroid 2010;20:1085–1093.
Niemeier LA, Kuffner Akatsu H, Song C, et al. A combined molecular-pathologic score improves risk stratification of thyroid papillary microcarcinoma. Cancer 2011;118:2069–2077.
Acknowledgements
We wish to acknowledge Monica Talmor for BRAFV600E mutational analysis, and Sarah Whitaker for her help with images.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This study was presented at the annual meeting of the United States and Canadian Academy of Pathology, Vancouver, 2012 as a poster.
Rights and permissions
About this article
Cite this article
Virk, R., Van Dyke, A., Finkelstein, A. et al. BRAFV600E mutation in papillary thyroid microcarcinoma: a genotype–phenotype correlation. Mod Pathol 26, 62–70 (2013). https://doi.org/10.1038/modpathol.2012.152
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2012.152
Keywords
This article is cited by
-
Integrating BRAFV600E mutation, ultrasonic and clinicopathologic characteristics for predicting the risk of cervical central lymph node metastasis in papillary thyroid carcinoma
BMC Cancer (2022)
-
Warum muss ein Schilddrüsentumor als Karzinom klassifiziert werden, wenn er sich biologisch nicht wie Krebs verhält?
Der Onkologe (2019)
-
Upregulation of the solute carrier family 7 genes is indicative of poor prognosis in papillary thyroid carcinoma
World Journal of Surgical Oncology (2018)
-
The increasing prevalence of chronic lymphocytic thyroiditis in papillary microcarcinoma
Reviews in Endocrine and Metabolic Disorders (2018)
-
BRAF and NRAS Mutations in Papillary Thyroid Carcinoma and Concordance in BRAF Mutations Between Primary and Corresponding Lymph Node Metastases
Scientific Reports (2017)