Abstract
Human epidermal growth factor receptor-2 (HER2) is a well-recognized growth-promoting factor in cancer, although its application to urothelial carcinoma has been limited because of a low frequency of gene amplification. We evaluated HER2 protein expression and gene amplification in micropapillary carcinoma, a rare but highly aggressive variant of urothelial carcinoma by dual-color in situ hybridization. Gene amplification was defined by a HER2:CHR17 ratio of ≥2.2; low and high levels of amplification were further defined as <2.5 and ≥2.5, respectively. Immunohistochemistry was used to determine HER2 protein expression using the American Society of Clinical Oncology/College of American Pathologists Guidelines of HER2 staining. Protein expression, gene amplification, and chromosome 17 aneusomy were compared by Jonchkeere–Terpstra and Cochran–Armitage trend tests. In all, 19 of the 20 micropapillary carcinoma samples yielded usable dual-color in situ hybridization and immunohistochemistry results for evaluation. Overall, 68% (n=13) demonstrated HER2 protein expression of 2+ to 3+ staining. Gene amplification was present in 42% of samples (n=8), with 100% correlation with 2+ and 3+ protein expression. Gene amplification and protein expression were significantly associated (P=0.01). Overall, 53% of samples (n=10) had aneusomy of chromosome 17. Chromosome 17 aneusomy was present in approximately half of the samples evaluated, suggesting inherent genomic instability in this variant of urothelial carcinoma. However, increased HER2:CHR17 ratios demonstrate increased HER2 expression due to amplification in the majority of micropapillary carcinomas. These results suggest that HER2-targeted therapy may be successful on the genomic level in patients with this disease.
Similar content being viewed by others
Main
The micropapillary variant of urothelial carcinoma (micropapillary carcinoma) is a rare but aggressive subtype of urothelial carcinoma. Histologically, micropapillary carcinoma resembles papillary serous carcinoma of the ovary with filiform projections that lack a central vascular core and are present within prominent retraction spaces.1 Although micropapillary carcinoma represents only 0.7–2.2% of bladder cancers,1, 2, 3 patients with this form of carcinoma often present at an advanced stage with muscle-invasive or metastatic disease2, 4 and a significantly reduced survival as compared with conventional urothelial carcinoma. Even when patients present with non-muscle-invasive disease, subsequent pathological upstaging occurs in up to 75% of patients and occult lymph-node disease may be present in 38% of patients.1 It is unclear whether the aggressive nature of micropapillary carcinoma is caused by its rapid growth rate or by its inherent biologically aggressive behavior.5, 6 Treatment options are usually limited to surgical removal of the bladder (cystectomy), as these tumors often demonstrate a poor response to intravesical treatment or chemotherapy.1, 5 As a result, other options for therapy are required to successfully address this lethal disease.
The human epidermal growth factor receptor-2 (ERBB2; HER2) is a transmembrane tyrosine kinase receptor the overexpression of which has been identified in multiple cancers, including breast, ovarian, salivary gland, endometrial, pancreatic, and non-small cell lung cancer.7, 8, 9 HER2 is normally responsible for regulating cell proliferation and survival.9, 10 Its overexpression results in increased vascular endothelial growth factor production and thus angiogenesis,11 as well as increased cell proliferation and decreased cell–cell adhesion by activation of the ras/mitogen-activated protein kinase pathway,11, 12 which are important factors in oncogenesis and tumor progression.8, 9, 10 HER2 protein overexpression has been most extensively researched in breast cancer, in which it occurs in ∼20% of primary invasive carcinomas13 and portends a poor prognosis with decreased disease-free and overall survival.8, 14 Encouragingly, there has been successful targeting of HER2-positive cells (defined as 2+ and 3+ protein expression with concurrent amplification) in metastatic breast cancer, resulting in longer overall survival and a 20% reduction in the risk of death when the anti-HER2 antibody trastuzumab (Herceptin; Genetech) is combined with standard chemotherapy.15
On the basis of these findings, there has been interest in the possible efficacy of HER2-targeted therapy in other cancers. Previous investigation into HER2 expression in conventional urothelial carcinoma has identified an inconsistent and often low frequency of gene amplification in this disease (0–32% of cases16, 17, 18),which made HER2-targeted therapy less feasible. However, there is a paucity of literature on HER2 expression in micropapillary carcinoma. As micropapillary carcinoma demonstrates a distinctive morphology and behavior from conventional urothelial carcinoma, we hypothesized that such differences could be an effect of differences in growth-promoting factors such as HER2. The goal of this study was to evaluate HER2 protein expression and HER2 gene amplification in this unique subset of urothelial carcinoma patients to determine whether HER2-targeted systemic therapy would provide a novel treatment option in this population, especially given the aggressiveness of this disease and the potential for metastatic disease by the time of presentation.2, 4
Materials and methods
Tissue Samples
This study was performed after obtaining approval by the Institutional Review Board. In all, 20 patients were identified with micropapillary carcinoma by pathological evaluation after undergoing transurethral resection or radical cystectomy at our institution between the years 1998 and 2008. All pathology slides were re-evaluated to confirm the initial diagnosis. Specimens were processed as a tissue microarray containing three to four 1.0-mm cores from separate regions of each tissue sample to assess for heterogeneity of protein and gene expression. We averaged our findings among the difference cores from separate tumor sites to address heterogeneity. All cores chosen demonstrated a majority of micropapillary features. Cores that were simply fat or did not represent carcinoma were excluded. Normal tissue samples were also included for reference and analysis, including tonsil, kidney, liver, small intestine, placenta, and lung. Tissue microarray samples were analyzed for HER2 expression by subsequent immunohistochemical staining and HER2 gene amplification by color dual hapten silver in situ hybridization (dual-color in situ hybridization).
Immunohistochemistry
Immunohistochemical stains for HER2 were performed using PATHWAY HER-2/neu (4B5) Primary Antibody (Ventana Medical Systems Inc., Tucson, AZ) as per the standardized Ventana PATHWAY HER2/neu protocol. Staining was performed on 3–4 μm tissue microarray sections, and only membrane staining was evaluated. Protein expression was scored according to the American Society of Clinical Oncology/College of American Pathologists guidelines19 of HER2 staining, defined as 0 (no staining), 1+ (incomplete membrane staining), 2+ (complete but weak membrane staining in >10% of cells), and 3+ (intense membrane staining in >30% of cells).18 Scoring was performed independently by two different reviewers (DEH and CBC). Positive control samples accompanying the micropapillary cell carcinoma samples were used for reference.
Consistent with other published studies of HER2 immunohistochemical staining in urothelial carcinoma,9, 12, 13, 15, 20 we considered carcinomas positive if they exhibited 2+ or 3+ immunostaining.
Dual-color in situ hybridization analysis for HER2 gene amplification and aneusomy
Gene amplification of HER2 was assessed by dual-color in situ hybridization—a form of chromogenic in situ hybridization. As compared with fluorescent in situ hybridization, dual-color in situ hybridization uses a combination of enzyme metallography and alkaline phosphatase-based chromogenic in situ hybridization to detect a dinitrophenyl haptenated repeat depleted HER2 probe and a digoxigenin haptenated repeat depleted chromosome 17 α-centromeric reference probe, respectively. HER2 gene copies (discrete black metallic silver signals) and chromosome 17 (CHR17) reference gene signals (red) are identified and enumerated by conventional bright-field microscopy after hematoxylin counterstaining.21, 22 All dual-color in situ hybridization procedural steps were fully automated on the BenchmarkXT workstation (Ventana Medical Systems Inc.). A single reviewer (CBC) evaluated each tissue microarray to determine aneusomy and HER2 gene amplification using a bright-field microscope (Olympus BX41, Olympus America, Center Valley, PA). Only cells in which at least two CHR17 reference probe signals could be identified on section were included for review. Cells were considered positive for aneusomy if >2 CHR17 probes per cell were identified and positive for HER2 gene amplification if the HER2:CHR17 ratio ≥2.2. Low-level amplification was defined as a ratio of 2.2 to <2.5 and high-level amplification as ≥2.5. At least 30 different randomly selected nuclei (range from 38–212 nuclei per specimen) from various specimen regions were selected for evaluation. Dual-color in situ hybridization results were then averaged to determine overall gene amplification levels for each specimen.
Patient information
Chart review was performed to obtain patient information and determine overall patient survival. The Social Security Death Index (http://ssdi.rootsweb.ancestry.com/) was also used to cross-reference patient status. Patient follow-up was considered until the time of death either as documented in the hospital chart or as recorded in the social security death index, or was considered the date of last follow-up in the medical chart.
Statistical Analysis
HER2 protein expression, HER2 gene amplification, and aneusomy 17 were summarized as frequency counts and percentages; and the Cochran–Armitage trend test and Jonchkheere–Terpstra tests were used to examine associations between them. The log-rank test was used to compare overall survival between groups. Data were analyzed using SAS 8.0 (SAS Institute Inc., Cary, NC) and StaXact 7.0 (Cytel Inc., Cambridge, MA).
Results
Patient Characteristics
Of the 20 micropapillary carcinoma patients evaluated, the majority were men (16 men; 4 female), consistent with the recognized distribution of this disease.5, 23 The mean patient age was 68 years (median 70 years, range 50–89 years). The majority of specimens were obtained after radical cystectomy with or without previous transurethral resection; four of the specimens were from transurethral resection alone (Table 1). In all, 19 of the 20 total samples met staining criteria for study inclusion and were used for sample evaluation and subsequent analysis.
All but two patients demonstrated evidence of muscularis propria invasion on pathological evaluation, with nearly all cystectomy specimens showing gross perivesical fat invasion. In all, 10 of the 17 patients with muscle-invasive disease died of disease, whereas 7 were still alive as of the last follow-up. In all, 14 patients had regional lymph-node metastases discovered at cystectomy. One of the patients undergoing transurethral resection of a bladder tumor lacked muscularis propria in the specimen, whereas a second showed uninvolved muscularis propria on sampling. Both of these patients showed adenopathy on imaging and subsequently died of the disease.
Overall, 10 patients had documented metastatic disease detected at follow-up after surgery, including enlarging lymph nodes on imaging (n=5), peritoneal carcinomatosis (n=1), brain metastases (n=1), liver metastases (n=1), lung metastases (n=1), or retroperitoneal tumor recurrence (n=1). Five patients did not have further imaging post-operatively, and four patients did not have any evidence of metastases on post-operative follow-up imaging. In all, 11 patients received chemotherapy, all post-operatively, with the majority (10 patients) receiving gemcitabine/cisplatin. The median overall survival was 13.8 months. A total of 12 patients died of disease. The remaining seven patients were alive as of last follow-up, with follow-up ranging from 0.2 to 37.4 months (mean 18.5 months; median 22.4 months). In three of the seven living patients, follow-up was <1 year. When evaluating the potential role chemotherapy had on survival, there was no association (P=0.56).
HER2 Protein Expression Occurs in the Majority of Micropapillary Carcinomas
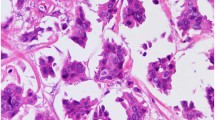
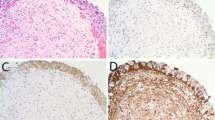
Evaluation of HER2 protein expression was performed by immunohistochemistry and was scored as described under the ‘Materials and methods’ section (Figure 1a–h). Protein expression was considered to be an immunohistochemistry score of 2+ or 3+. Overall, 13 patient samples (68%) demonstrated HER2 protein expression, including 8 patients with 2+ immunohistochemistry and 5 patients with 3+ immunohistochemistry (Table 2). No significant association was present between HER2 protein expression and overall survival (P=0.28; Figure 2a).
Association of HER2 and aneusomy status with overall survival. (a) HER2 protein expression, stratified into 0–1+ and 2–3+ categories, did not significantly correlate with overall survival (P=0.28). (b) Similarly, overall survival was not significantly associated with HER2 gene amplification, regardless of low or high levels of amplification (P=0.53). (c) A trend toward decreased survival with aneusomy 17 was apparent (P=0.07).
HER2 Gene Amplification is Present in Nearly Half of the Micropapillary Carcinomas and Correlates with Protein Expression
Dual-color in situ hybridization was used to determine HER2 copy number with the CHR17 probe used for standardization. HER2 gene amplification, defined as a ratio of HER2:CHR17 of ≥2.2, was identified in 8 specimens (42%; Figure 3a–c). Gene amplification was also further stratified into low- and high-level amplification. In particular, low-level amplification was defined as a HER-2:CHR17 ratio ≥2.2 and <2.5 and high-level amplification was defined as a HER-2:CHR17 ratio of ≥2.5. Gene amplification was only present in samples with protein expression; none of the specimens with 0 to 1+ staining demonstrated gene amplification (100% concordance). Both overall gene amplification and amplification defined as none, low, and high, were significantly related to protein expression (P=0.01 and P<0.01, respectively, Tables 2 and 3). Of the five samples with 3+ HER2 protein expression, three demonstrated high-level gene amplification; conversely, of the eight samples with 2+ HER2 protein expression, only one had high-level amplification. No significant association was identified between HER2 gene amplification and overall survival (P=0.53; Figure 2b).
Dual-color in situ hybridization analysis was used to evaluate HER2 gene amplification (black) and aneusomy of chromosome 17 (red). (a) A subset of cases demonstrated no HER2 amplification or aneusomy, with a 1:1 ratio of HER2:CHR17 probe and two CHR17 copies per cell. (b) Aneusomy 17 occurred in a high proportion of cases and is reflected in the presence of >2 centromeric/CHR17 probes. (c) HER2 gene amplification is reflected by a HER2:CHR17 ratio of ≥2.2 (arrow).
Aneusomy 17 is Frequent in Micropapillary Carcinoma and May Affect Patient Outcome
The CHR17 probe was used to evaluate aneusomy of chromosome 17. Of the 19 samples evaluated, aneusomy was present in 10 cases (52.6%). No significant association was identified between aneusomy and HER2 protein expression or gene amplification (P=0.27 and P=0.41, respectively). However, there is some suggestion that patients with aneusomy 17 may have a reduced overall survival relative to those with normal chromosome 17 numbers (P=0.07; Figure 2c). Given that all but three of the patients had evidence of metastatic disease, either by pathological review or upon imaging, an association between metastatic spread and aneusomy 17 amplification could not be accurately assessed. Similarly, the joint impact of aneusomy 17 and HER2 amplification could not be accurately assessed because of the low number of patients in these categories (Table 4).
Discussion
Micropapillary carcinoma represents a distinctive subtype of urothelial carcinoma. A defining feature is the aggressive biological behavior of this lesion, which may represent a difference in tumor cell growth or invasiveness.4, 24, 25 Tumors that demonstrate an admixture of conventional urothelial carcinoma and micropapillary carcinoma often demonstrate metastases composed of the micropapillary carcinoma component, supporting the aggressive nature of this variant.4, 24, 25 Even in primary tumors, the percentage of micropapillary carcinoma present is significantly related to the stage of disease and mortality:25 a relative mortality risk of 2.4 is present when the component of micropapillary carcinoma is >50% as compared with those with pure conventional urothelial carcinoma or <50% micropapillary carcinoma.26 One of the first reports of micropapillary carcinoma found that 94% of patients had muscle-invasive disease at the time of presentation, with frequent angiolymphatic invasion.4 A more recent review found that 91% of patients had at least clinical T1 disease at presentation.1
The exact mechanism by which micropapillary carcinoma pathology differs from that of conventional urothelial carcinoma is unknown. One suggestion is that its aggressive nature may be due to a reversal in cell polarity, resulting in the basal surface acquiring apical secretory properties that promote tumor invasion.23, 27 There is also evidence to suggest a high level of inherent chromosomal or genomic instability in micropapillary carcinoma: micropapillary carcinoma contains a higher amount of DNA content manifested by a DNA index of 1.75 for invasive micropapillary carcinoma as compared with 1.5 for noninvasive papillary urothelial carcinoma. The index increased to 1.85 for metastatic micropapillary carcinoma.4, 24 In another study, expressions of known predictors of poor prognosis—including p53, MIB-1, Aurora-A, and survivin—are increased in micropapillary carcinoma as compared with conventional urothelial carcinoma.28 In particular, Aurora-A is a known activator of genetic pathways involving centrosome amplification and chromosomal missegregation, resulting in increased chromosome copy number and nuclear DNA content.29
The HER2 protein is considered to have a significant role in bladder cancer outcomes, with HER2 protein expression reported in up to 31–65.5% of samples.12, 16, 30 Furthermore, HER2 protein expression seems to correlate with tumor stage, tumor grade,30, 31, 32 and poor prognosis.30, 31, 32, 33, 34 One study found that the 5-year disease-free survival rate decreased from 48.5% in HER2-negative patients to 9.7% in those who were HER2 positive.34 Skagias et al33 also found that HER2 expression correlated with decreased disease-specific survival (P=0.002) and overall survival (P=0.025). Previous analysis of HER2 gene amplification in conventional urothelial carcinoma found that gene amplification was present in only 10–11% of metastatic and non-metastatic lesions9 and HER2 protein expression in only 30–36% of cases. Overall, there does not seem to be a strong association between HER2 protein expression and gene amplification in conventional urothelial carcinoma,12 which is perhaps explained by the relative paucity of gene amplification found in conventional urothelial carcinoma specimens, ranging from only 5.1 to 22.2%.12, 16, 17, 18 Sauter et al35 found that even when urothelial carcinomas show HER2 amplification, there is extreme heterogeneity in the amount of amplification per cell, with areas of amplification ‘clustering’ together and the fraction of amplified cells ranging from 18–94% within tumors.
The current study of the micropapillary carcinoma variant demonstrates HER2 protein expression (2+ to 3+) and gene amplification in a significantly higher proportion of cases—68 and 42%, respectively. In support of these findings, a separate study that compared HER2 protein expression between micropapillary carcinoma and conventional urothelial carcinoma found a similar discrepancy, with 25% of invasive micropapillary carcinoma samples staining 3+ for HER2 protein as compared with 8% in invasive urothelial carcinoma with retraction artifact, although gene amplification—which mediates response to HER2 mediated therapy—was not evaluated.36 In our study, we also identified potential chromosomal and genomic instability in micropapillary carcinoma, including the presence of aneusomy 17 in over half (52.6%) of our samples. As the HER2 gene is located on 17q11–21,12, 20 HER2 protein expression has been often linked to aneusomy of chromosome 17—one of the most common aberrations in conventional urothelial carcinoma.37 As a result, studies have often found a 100% correlation between HER2 protein expression and aneusomy of chromosome 17.12, 30 However, the finding of HER2 gene amplification in a large proportion of our micropapillary carcinoma cases suggests an additional mechanism for HER2 protein expression in this urothelial carcinoma variant and suggests the potential relevance of HER2 in micropapillary carcinoma biology and therapy.
The frequent finding of HER2 gene amplification in our micropapillary carcinoma study is particularly encouraging, given that gene amplification is important for controlling response to HER2-targeted therapy.8 Although protein expression can be used as a surrogate to determine gene amplification, it does not reliably predict the response to HER2-directed therapy.38 However, in some cancers, HER2 gene amplification and protein expression correlate well, such as in breast, ovarian, and gastric cancer,14, 39, 40 with HER2 gene amplification found in >90% of breast cancer patients demonstrating HER2 protein overexpression.8 In our study, the significant association between protein expression and gene amplification suggests that HER2 protein analysis may also provide a surrogate marker for gene amplification in micropapillary carcinoma. It should be noted that not all samples with protein expression had gene amplification suggesting other mechanisms leading to overexpression. Suggestions for these findings include protein expression due to transcriptional or post-transcriptional dysregulation.35, 41
Although there have not been any studies evaluating HER2-targeted therapy in micropapillary carcinoma, several trials have investigated its role in conventional urothelial carcinoma. Unfortunately, these studies have had limited success, likely because of the low rates of gene amplification in this population. In a phase II study of a dual inhibitor of HER-1 and HER2 kinase, only 1.7% of locally advanced or metastatic UCC patients had an objective response.16, 42 A separate phase II trial that combined trastuzumab (monoclonal antibody to HER2) with carboplatin, paclitaxel, and gemcitabine showed a complete response in 5 of 44 (11.4%) HER2-positive patients and a partial response in 26 of 44 (59.1%) patients (defined as a reduction of ≥50% in the size of the lesion).43 Although many of these studies used HER2 protein expression as a marker for potential therapeutic response, the lack of close correlation between protein levels and gene amplification and the low number of conventional UCCs that show HER2 gene amplification may explain these results. In contrast, the high frequency of gene amplification in micropapillary carcinoma suggested by our findings may provide a rationale to further evaluate HER2-targeted therapy in an enriched population and particularly in those with metastatic disease.
It should be noted that there are differing definitions of amplification and overexpression. The American Society of Clinical Oncology/College of American Pathologists Guidelines—which are specifically geared towards HER2 expression in breast cancer—outlines gene amplification as HER2/CEP17 ratio >2.2 and protein overexpression as 3+ cell staining.19 However, The literature surrounding HER2 expression in urothelial cancer often defines overexpression as both 2+ and 3+ staining.9, 12, 13, 15, 20 Similarly, previous papers have defined gene amplification as a HER2/CEP17 ratio of >2. We have attempted to meld the definitions of previous literature with the guidelines outlined by the American Society of Clinical Oncology to make our paper comparable with previous studies but accurate with the universal definitions.
In conclusion, micropapillary carcinoma demonstrates a marked difference in HER2 expression from conventional UCC. The frequent finding of HER2 gene amplification in this population seems to drive HER2 protein expression, with which it is closely associated, and suggests a potential new means for targeted therapy. Frequent aneusomy of chromosome 17 in micropapillary carcinoma supports the possibility of increased genomic instability in this UCC variant, which may influence the ultimate behavior of these tumors.
References
Kamat AM, Dinney CPN, Gee JR, et al. Micropapillary bladder cancer. Cancer 2007;110:62–67.
Johansson SL, Borghede G, Holmang S . Micropapillary bladder carcinoma: a clinicopathological study of 20 cases. J Urol 1999;161:1798–1802.
Trabelsi A, Stita W, Soumaya R, et al. Micropapillary carcinoma of the urinary bladder: a case report and review of the literature. CUAJ 2008;2:540–542.
Amin MB, Ro JY, el-Sharkawy T, et al. Micropapillary variant of transitional cell carcinoma of the urinary bladder. Histologic pattern resembling ovarian papillary serious carcinoma. Am J Surg Pathol 1994;18:1224–1232.
Kamat AM, Gee JR, Dinney CP, et al. The case for early cystectomy in the treatment of nonmuscle invasive micropapillary bladder carcinoma. J Urol 2006;175:881–885.
Nishizawa K, Kobayashi T, Mitsumori K, et al. Micropapillary bladder cancer. Int J Urol 2005;12:506–508.
Tolmachev V . Imaging of HER2 over-expression in tumors for guiding therapy. Curr Pharm Des 2008;14:2999–3019.
Scholl S, Beuzeboc P, Pouillart P . Targeting HER2 in other tumor types. Ann Oncol 2001;12:S81–S87.
Hansel DE, Swain E, Dreicer R, et al. HER2 over-expression and amplification in urothelial carcinoma of the bladder is associated with MYC coamplification in a subset of cases. Am J Surg Pathol 2008;130:274–281.
Eissa S, Ali HS, Al Tonsi AH, et al. HER2/neu expression in bladder cancer: relationship to cell cycle kinetics. Clin Biochem 2005;38:142–148.
Blackwell KL, Dewhirst MW, Liotcheva V, et al. HER2 gene amplification correlates with higher levels of angiogenesis and lower levels of hypoxia in primary breast tumors. Clin Cancer Res 2004;10:4083–4088.
Caner V, Turk NS, Duzcan F, et al. No strong association between HER2/neu protein over-expression and gene amplification in high-grade invasive urothelial carcinoma. Pathol Oncol Res 2008;14:261–266.
Gandour-Edwards R, Lara Jr PN, Folkins AK, et al., DeVere-White R Does HER2/neu expression provide prognostic information in patients with advanced urothelial carcinoma? Cancer 2002;95:1009–1015.
Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER2/neu oncogene. Science 1987;235:177–182.
Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783–792.
Lae M, Couturier J, Oudard S, et al. Assessing HER2 gene amplification as a potential target for therapy in invasive urothelial bladder cancer with a standardized methodology: results in 1005 patients. Ann Oncol 2010;21:815–819.
Underwood M, Bartlett J, Reeves J, et al. C-erbB-2 gene amplification: a molecular marker in recurrent bladder tumors? Cancer Res 1995;55:2422–2430.
Schraml P, Kononen J, Bubendorf L, et al. Tissue microarrays for gene amplification surveys in many different tumor types. Clin Cancer Res 1999;5:1966–1975.
Wolff AC, Hammond EH, Schartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 2007;25:118–145.
Latif Z, Watters AD, Dunn I, et al. HER2/neu over-expression in the development of muscle-invasive transitional cell carcinoma of the bladder. Br J Cancer 2003;89:1305–1309.
Nitta H, Hauss-Wegrzyniak B, Lehrkamp M, et al. Development of automated bright field double in situ hybridization (BDISH) application for HER2 and chromosome 17 centromere for breast carcinomas and an assay performance comparison to manual dual color HER2 fluorescence in situ hybridization (FISH). Diagn Pathol 2008;3:41.
Gruver AM, Peerwani Z, Tubbs RR . Out of the darkness and into the light: bright field in situ hybridization for delineation of ERBB2 (HER2) status in breast carcinoma. J Clin Pathol 2010;63:210–219.
Perepletchikov AM, Parwani AV . Micropapillary urothelial carcinoma: clinico-pathologic review. Pathol Res Pract 2009;205:807–810.
Amin MB . Histological variants of urothelial carcinoma: diagnostic, therapeutic and prognostic implications. Mod Pathol 2009;22:S96–S118.
Samaratunga J, Khoo K . Micropapillary variant of urothelial carcinoma of the urinary bladder: a clinicopathological and immunohistochemical study. Histopathology 2004;45:55–64.
Alvarado-Cabrero I, Sierra-Santiesteban FI, Mantilla-Morales A, et al. Micropapillary carcinoma of the urothelial tract: a clinicopathologic study of 38 cases. Ann Diagn Pathol 2005;9:1–5.
Luna-More S, Gonzalez B, Acedo C, et al. Invasive micropapillary carcinoma of the breast. A new special type of invasive mammary carcinoma. Pathol Res Pract 1994;190:668–674.
Comperat E, Roupret M, Conort P, et al. Aurora-A/STK-15 is differentially expressed in the micropapillary variant of bladder cancer. Urologia Internationalis 2008;82:312–317.
Sen S, Zhou H, Zhang RD, et al. Amplification/overexpression of a mitotic kinase gene in human bladder cancer. J Natl Cancer Inst 2002;94:1320–1329.
Simonetti S, Russo R, Ciancia G, et al. Role of polysomy 17 in transitional cell carcinoma of the bladder: immunohistochemical study of HER2/neu expression and fish analysis of c-erbB-2 gene and chromosome 17. Int J Surg Pathol 2009;17:198–205.
Li B, Kanamaru H, Noriki S, et al. Numeric aberration of chromosome 17 is strongly correlated wtih p53 over-expression, tumor proliferation and histopathology in human bladder cancer. Int J Urol 1998;5:317–323.
Inoue T, Sato K, Tsuchiya N, et al. Numeric aberrations of HER2 and chromosome 17 detected by fluorescence in situ hybridization in urine-exfoliated cells from patients with urothelial carcinoma. Urology 2004;64:617–621.
Skagias L, Politi E, Karameris A, et al. Prognostic impact of HER2/neu protein in urothelial bladder cancer. Survival analysis of 80 cases and an overview of almost 20 years' research. J BUON 2009;14:457–462.
Sato K, Moriyama M, Mori S, et al. An immunohistologic evaluation of c-erbB-2 gene product in patients with urinary bladder carcinoma. Cancer 1992;70:2493–2499.
Sauter G, Moch H, Moore D, et al. Heterogeneity of erbB-2 gene amplification in bladder cancer. Cancer Res 1993;53:2199–2203.
Sangoi AR, Higgins JP, Rouse RV, et al. Immunohistochemical comparison of MUC1, CA125, and HER2Neu in invasive micropapillary carcinoma of the urinary tract and typical invasive urothelial carcinoma with retraction artifact. Mod Pathol 2009;22:660–667.
Hovey RM, Chu L, Balazs M, et al. Genetic alterations in primary bladder cancers and their metastases. Cancer Res 1998;58:3555–3560.
Mass RD, Press MF, Anderson S, et al. Evaluation of clinical outcomes according to HER2 detection by fluorescence in situ hybridization in women with metastatic breast cancer treated with trastuzumab. Clin Breast Cancer 2005;6:240–246.
Hynes NE, Stern DF . The biology of erbB-2/neu/HER2 and its role in cancer. Biochem Biophys Acta Rev Cancer 1994;1198:165–184.
Press MF, Cordon-Cardo C, Slamon DJ . Expression of the HER2/neu proto-oncogene in normal human adult and fetal tissues. Oncogene 1990;5:953–962.
Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER2/neu proto-oncogene in human breast and ovarian cancer. Science 1989;244:707–712.
Wulfing C, Machiels JP, RIchel DJ, et al. A single-arm, multicenter, open-label phase 2 study study of lapatinib as the second-line treatment of patients wtih locally advanced or metastatic transitional cell carcinoma. Cancer 2009;115:2881–2890.
Hussain MHA, MacVicar GR, Petrylak DP, et al. Trastuzumab, paclitaxel, carboplatin, and gemcitabine in advanced human epidermal growth factor receptor-2/neu-positive urothelial carcinoma: results of a multicenter phase II National Cancer Institute trial. J Clin Oncol 2007;25:2218–2224.
Acknowledgements
Reagents for immunohistochemistry and dual-color in situ hybridization were provided by Ventana Medical Systems Inc., Tucson, AZ.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Application of PATHWAY HER-2/neu (4B5) Primary Antibody in bladder tissue is outside the approved intended use. The HER2 dual-color in situ hybridization reagents were for research use only.
Rights and permissions
About this article
Cite this article
Ching, C., Amin, M., Tubbs, R. et al. HER2 gene amplification occurs frequently in the micropapillary variant of urothelial carcinoma: analysis by dual-color in situ hybridization. Mod Pathol 24, 1111–1119 (2011). https://doi.org/10.1038/modpathol.2011.69
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2011.69
Keywords
This article is cited by
-
Divergent differentiation and variant morphology in invasive urothelial carcinomas – association with muscle-invasive disease
Surgical and Experimental Pathology (2020)
-
Variant morphology and random chromosomal integration of BK polyomavirus in posttransplant urothelial carcinomas
Modern Pathology (2020)
-
Genomic heterogeneity in bladder cancer: challenges and possible solutions to improve outcomes
Nature Reviews Urology (2020)
-
Clinicopathologic and Immunohistochemical Study of Combined Small Cell Carcinoma and Urothelial Carcinoma Molecular Subtype
Pathology & Oncology Research (2019)
-
SIU–ICUD on bladder cancer: pathology
World Journal of Urology (2019)