Abstract
The concept of a “p53 null phenotype” (complete loss of staining) is well-recognized in the gynecologic pathology literature, implicitly reflecting that this staining pattern represents a TP53 mutation. However, in the genitourinary pathology literature, a p53 null phenotype has only been addressed regarding the prognosis of invasive urothelial carcinoma, and not as a diagnostic biomarker for urothelial carcinoma in situ (CIS). Herein, 25 cases of urothelial carcinoma in situ [diagnoses made on hematoxylin and eosin (H&E) stained sections] showing null pattern p53 staining were retrieved from 22 different patients (16 males and 6 females, age range 52–85 years; average 69.6 years), most commonly showing large cell pleomorphic pattern morphology. One representative tissue block per case was selected for next-generation DNA sequencing (NGS). All 21 cases (100%) passing quality control for NGS showed at least 1 TP53 mutation (majority nonsense or frameshift mutations), including 3 cases with 2 mutations and 3 cases with 3 mutations. Three patients with multiple available samples harbored 1 or more shared TP53 mutations at 2 different time points, indicating clonality of the temporally distinct lesions. Additionally, 2 patients had an additional unique TP53 mutation at a later time point, suggesting intratumoral heterogeneity and/or temporal clonal evolution. While urothelial CIS remains an H&E diagnosis in most cases, a p53 immunostain may be useful in a subset of challenging cases. This study demonstrates that a p53 null phenotype represents an aberrant result in urothelial CIS with supportive molecular analysis showing a previously unknown level of complexity for TP53 mutations among these noninvasive lesions. Adequate recognition of the p53 null phenotype as a “biologically supportive result”, similar to strong and diffuse staining with p53, is important and may warrant a formal consensus statement for recommended p53 reporting (i.e., “wild type” versus “aberrant or mutant”).
Similar content being viewed by others
Introduction
TP53 is a tumor suppressor gene located on chromosome 17 that functions as an important regulator of cell cycle, apoptosis, and genomic stability1. Immunohistochemical expression of p53 acts as a surrogate marker for TP53 mutations2,3, which are frequent in urothelial carcinomas4. Various cut-offs for “positive or aberrant” (i.e., mutant) p53 immunostaining have been proposed in urothelial carcinoma, ranging from ≥10% to 50%5,6,7,8, with one best practice recommendation employing the descriptor “strong and intense positivity”, as seen in urothelial CIS9. In the gynecologic pathology literature, a “null phenotype” (complete loss of staining) is well-recognized as a “positive or aberrant” result in the diagnostic immunohistochemical assessment of p53 expression, which in part may be due to frequency of encountering null phenotype p53 immunostaining in this context (up to 25% of tubo-ovarian serous carcinomas10). Moreover, null phenotype and diffuse/strong patterns are both formally included as “abnormal or mutant” results in the recommended terminology for interpreting and reporting p53 immunohistochemistry10,11. However, in the genitourinary pathology literature, an investigation of a null phenotype p53 staining has mainly involved invasive urothelial carcinoma and has been evaluated as a prognostic indicator12, with limited studies focusing on this staining pattern in diagnosis13,14,15, and none, to our knowledge, exclusively on urothelial carcinoma in situ (CIS). Given our anecdotal experience with this staining pattern, including some p53 null phenotype CIS cases that were misinterpreted as wild type, we sought to formally examine and report a larger clinicopathologic series of such cases.
Methods and materials
Case Selection and p53 immunohistochemical analysis
The pathology archives of the authors’ institutions were searched for cases of urothelial CIS in which p53 immunohistochemistry yielded a null phenotype staining pattern (complete loss of p53 staining in lesional cells). Clone details for p53 stains which were performed at the authors’ institutions are summarized in Table 1. The diagnosis of urothelial CIS for each case was confirmed by at least 2 experienced genitourinary pathologists, based on hematoxylin and eosin (H&E) stained sections and standard morphologic criteria. One case misinterpreted as wild type p53 staining was included in the study. All cases with p53 null pattern staining in urothelial CIS had patchy weak staining in background benign urothelium (internal control). Clinicopathologic features of all cases were recorded, including results of keratin 20 (lesional cell reactivity considered positive result) and/or CD44 (absent staining in lesional cells considered positive result) immunohistochemical markers that were used. The morphologic pattern(s) of urothelial CIS [large cell with pleomorphism, large cell without pleomorphism, small cell, cancerization (pagetoid/undermining), clinging, or plasmacytoid15,16] were noted for all cases.
Next-generation DNA sequencing
Representative formalin-fixed paraffin-embedded (FFPE) tumor tissue was obtained from all cases for targeted next-generation DNA sequencing (DNAseq) which was performed at 2 institutions [19 cases from University of Michigan (UM) and 5 cases from University of California San Francisco (UCSF)].
At UM, unstained FFPE tumor tissue was manually scraped from glass slides via comparison to an adjacent H&E-stained section, and DNA was extracted using the AllPrep DNA/RNA FFPE Kit (Qiagen, Hilden, Germany). Extracted DNA was quantitated with a Qubit fluorometer (Thermo Fisher Scientific, Waltham, MA), and up to 20 ng of Uracil-DNA Glycosylase (UDG)-treated DNA was utilized to generate next-generation sequencing (NGS) libraries using the Ion AmpliSeqTM HD Library Kit (Thermo Fisher Scientific) and a custom Ion AmpliSeqTM HD panel (Thermo Fisher Scientific) that targets the entire coding sequence of the TP53 gene. NGS libraries were templated for sequencing using an Ion ChefTM Instrument (Thermo Fisher Scientific) and sequenced on an Ion GeneStudioTM S5 Prime System (Thermo Fisher Scientific) with the Ion 540TM Chip Kit (Thermo Fisher Scientific). NGS reads were aligned and processed using Torrent Suite Software (version 5.14; Thermo Fisher Scientific) and the coverageAnalysis, molecularCoverageAnalysis, and variantCaller plugins. NGS libraries with a median molecular coverage less than 50 were excluded from variant analysis. Variant Call Format (VCF) files were processed and annotated using established in-house bioinformatics pipelines, as well as the AmpliSeq HD for Tumor - w2.5 - DNA - Single Sample workflow using the cloud-based Ion Reporter Software (version 5.18.1.0; ionreporter.thermofisher.com/ir/; Thermo Fisher Scientific). Annotated nonsynonymous variants, splice site variants, and indels were prioritized using the following criteria: Phred QUAL Score > 60; Homopolymer Length < 5; and gnomAD allelic frequency < 0.001. Variants with a Phred QUAL Score ≤ 60 but which were prioritized in a separate sample from the same patient were also considered prioritized. Finally, all prioritized annotated variants were manually visualized using the Integrative Genomics Viewer (version 2.8.3; software.broadinstitute.org/software/igv/; Broad Institute, Cambridge, MA) for confirmation.
At UCSF, NGS was performed previously for clinical purposes in a CLIA-certified laboratory using the UCSF500 Cancer Gene Test, a clinically validated next generation sequencing assay or part of a previously published series17. At the time NGS was performed on these cases, the panel including sequencing of the entire coding regions of 479 cancer-related genes (including TP53) as well as select introns of 47 genes. Data was obtained from reviewing the original molecular reports in the electronic medical record.
Results
A total of 25 study cases showing null pattern p53 immunostaining were retrieved, from 22 different patients, including 16 males and 6 females, ages ranging from 52 to 85 years (average 69.6 years). Three patients underwent bladder biopsy at different time intervals (biopsy of the same bladder site in 2 of 3 patients). Three of the 25 cases (12%) also showed invasive urothelial carcinoma away from the CIS in the same tissue (all 3 cases were resections). The most common morphologic pattern of CIS was large cell pleomorphic (n = 18, 72%), followed by pagetoid (n = 5, 20%) including 3 cases showing two concomitant CIS patterns (either pagetoid or clinging admixed with large cell pleomorphic). Twenty-three cases had additional immunostains performed, with 23 of 23 (100%) showing diffuse strong keratin 20 and 13 of 13 (100%) showing loss of CD44. Fifteen cases presented as pTis stage at the time of diagnosis, with the remaining showing higher stage based on other tissue blocks or separate specimen sites submitted at initial diagnosis. Table 1 shows detailed clinicopathologic features of the study cases and Figs. 1 and 2 demonstrate representative images of the cases.
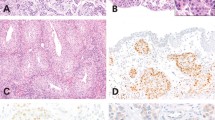
H&E of large cell pleomorphic pattern urothelial carcinoma in situ (A, 200×). Paired p53 immunostains showing null phenotype pattern (complete loss of staining in lesional cells) (B, 200×). Paired positive keratin 20 staining in lesional cells (C, 200×) and negative CD44 staining in lesional cells (D, 200×).
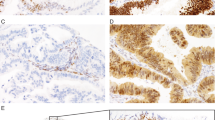
H&E of large cell pleomorphic pattern urothelial carcinoma in situ (A, 200×) and paired null phenotype p53 staining in lesional cells (B, 200×). H&E of large cell pleomorphic and clinging patterns urothelial carcinoma in situ (C, 200×) and paired null phenotype p53 staining in lesional cells (D, 200×). Perhaps based on patchy weak p53 staining in admixed benign admixed urothelial cells or because of unfamiliarity of the concept of “null phenotype” p53 immunoreactivity in urothelial neoplasia, p53 staining in this case was misinterpreted as wild-type. This case showed a frameshift deletion in TP53, supporting p53 staining as aberrant.
Clinical follow-up was available for 18 of 22 patients (82%), with 10 patients showing disease progression to higher stage (including 4 patients with eventual metastatic urothelial carcinoma), 6 patients showing persistence of CIS, 3 patients dying of disease, and 2 patients showing no evidence of disease.
Twenty-four of the 25 cases (96%) had FFPE tissue blocks available for targeted DNAseq of the TP53 gene. Of these, 21 cases (88%) passed the initial NGS quality control criteria for TP53 variant analysis (NGS performed at UM on 16 cases and at UCSF on 5 cases). All 21 cases showed at least one TP53 mutation, including 3 cases with 2 mutations, and 3 cases with 3 mutations. The majority (90%) of the TP53 mutations in these cases were nonsense or frameshift mutations, and all but one had at least one nonsense, splice site, or frameshift mutation. The one case without a nonsense, splice site, or frameshift mutation had two nonsynonymous mutations, including one hotspot mutation (p.C238Y) and one mutation at an amino acid position with other hotspot mutations (p.R290S). Three patients with multiple available samples harbored one or more shared TP53 mutations at 2 different time points, although in 2 of the 3 cases, an additional unique TP53 mutation was identified at the later time point. The large cell pleomorphic subtype showed p(Q192X) or pE198X mutation (3 and 2 cases respectively of 18 cases, see Table 1 for details) and the 2 cases with clinging subtype of CIS (which were admixed with large cell pleomorphic) showed pR213X mutation. The significance of this finding cannot be determined due to small number of cases in this cohort.
Discussion
In many instances, the diagnosis of urothelial CIS is straightforward and can be made without the use of immunohistochemistry. However, in some cases with subtle morphologic pattern of CIS15,16 (e.g., pagetoid, plasmacytoid) and in post-therapy settings, distinction of CIS from reactive urothelial atypia can be challenging. Several immunohistochemical markers have been studied to resolve this challenging differential diagnosis – including p53, keratin 20, CD44, AMACR, Ki67, p16, keratin 5/6, and Her2 – with p53, keratin 20, and CD44 emerging as recommended stains for potential utility9. Meta-analysis of 12 large urothelial CIS immunohistochemical studies that included p53 staining13,14,15,18,19,20,21,22,23,24,25,26 showed that only 3 studies either described or considered the p53 null pattern as mutant, with only 1 study specifying how many of their mutant cases fell into this category13,14,15. Of note, 1 study included semi-quantitative scoring for p53 immunohistochemistry, with 0 to 1+ staining designated p53 wild type, and 2+ or 3+ staining as p53 mutant26. Another study scored “no staining or predominantly basally located positivity” for p53 as wild type18, both contributing to artifactually lower p53 immunostain mutant rates. Interestingly, among all studies, the cut-off criteria for a mutant p53 immunohistochemistry was quite variable, with some including only descriptors (“strong” or “intense full thickness”), while others used numerical thresholds (ranging from 20% to 85%)5, 15-25. Only 4 of the 12 studies employed “wild type” p53 terminology in describing cases not deemed aberrant/abnormal13,14,15,20.
All p53 null cases with interpretable NGS results included in this study harbored at least 1 TP53 mutation, and all but 1 case showed either nonsense, splice site, or frameshift mutation – which are predicted to result in a truncated and/or substantially altered p53 protein product. These results corroborate the interpretation that the absent p53 staining in the lesional cells is truly aberrant (null TP53 mutation resulting in complete absence of detectable protein), a phenomenon described in gynecologic carcinomas10,27. The current CIS study targeted only abnormal p53 cases; to our knowledge, only one other study formally described molecular findings in urothelial CIS, reporting TP53 mutations in 12 of 15 cases (72%)28 – although that study predated the era of utilization of highly sensitive NGS technique. In addition to confirming the presence of TP53 mutations in CIS cases with a p53 null phenotype, our data reveal a previously unknown complexity of TP53 mutations in these noninvasive lesions. Six cases (29%) harbored more than one TP53 mutation, including 3 with 2 mutations, and 3 with 3 mutations. Interestingly, while 5 of the cases with more than one mutation had at least one nonsense mutation – including 2 cases with 2 nonsense mutations – none of these had a frameshift mutation. Although the precise reason for this observation is unclear – and our study was not designed or sufficiently powered to answer this question – these data suggest that there may be distinct pathogenic mechanisms underlying the acquisition of these mutations in urothelial CIS. Finally, all 3 patients with multiple available samples harbored one or more shared TP53 mutations at 2 different time points, indicating clonality of such metachronous lesions. In addition, 2 of the patients had an additional unique TP53 mutation at the later time point, suggesting intratumoral heterogeneity and/or temporal clonal evolution of CIS.
Although various factors, including some clinical ones, may contribute to disease progression in patients with urothelial CIS, some studies have shown that overexpression of p53 by immunohistochemistry correlates with the disease progression29,30. Of the 15 patients in our cohort that had pTis staging at the diagnosis, 6 patients had persistence of urothelial CIS (including 1 requiring cystectomy for non-responsiveness to therapy), and 10 patients had disease progression to higher stage, including 4 with metastatic disease. More recently, some studies have shown that p53 may have a role in the genesis of urothelial CIS, stratifying cases into basal and luminal subgroups, which may be prognostic31.
While p53 null pattern has been reported in 15% of all urothelial carcinomas12, data regarding the incidence of this staining pattern in urothelial CIS is limited (7% in one study14). In the authors’ own experience, as well as during the case collection among the participating genitourinary pathologists, null phenotype p53 immunostaining was infrequently encountered in the urothelial CIS. While we emphasize that urothelial CIS remains a morphologic diagnosis using only H&E stain in the majority of cases, we acknowledge the utility of p53 immunohistochemistry in a subset of challenging cases (often specific but with low sensitivity for CIS14,18), which may be helpful for the diagnosis in the appropriate context. In this regard, we have encountered challenging cases submitted for consult reviews, in which the pathologist was favoring a reactive urothelial atypia diagnosis based on a “wild type” p53 immunostaining, which in fact represented a “null phenotype” p53 immunostaining that in and (in conjunction with morphology) instead supported a urothelial CIS diagnosis. The goal of this study however was not necessarily to advocate for p53 immunohistochemistry as a sole marker or a superior one to keratin20 and CD44 immunostains. In fact, during the course of the study, it became evident that the utility and the practice of performing p53 immunohistochemistry varied greatly among the participants. Moreover, as a comparative assessment from reactive urothelial atypia was not the focus of this study, it is uncertain whether null phenotype p53 stain is exclusively seen in CIS (only one comparative study to our knowledge did not find p53 null pattern in reactive atypia14). While none of the study participants recalled encountering cases of null phenotype p53 in reactive urothelial atypia (personal communication by ARS), further investigation on the diagnostic specificity would be of interest.
Based on our collective experience of evaluating in-house cases and a high-volume secondary reviews, we conclude that aberrant p53 (along with keratin 20) immunostaining is prevalent in urothelial CIS, and that null pattern p53 immunostaining is under-appreciated in the day-to-day practice. The findings from this study necessitate standardization of p53 immunostaining terminology in genitourinary pathology reporting in the work-up of urothelial CIS cases, reflecting the importance of recognizing the pattern of “all (i.e., >80% of tumor cells strong and diffusely positive) or nothing staining”10,11 from the gynecologic pathology practice. Recognizing either intense nuclear positivity in almost all dysplastic urothelial cells or a “null phenotype”, as described in this study (i.e., lacking p53 expression in all cells) as “positive or aberrant”, has also been endorsed by the Genitourinary Pathology Society (GUPS) working group32. For example, a uniform terminology can be applied when signing out such cases as p53 “mutant-positive” or “mutant-null phenotype”. Moreover, a recommended cut-off percentage may be helpful for cases with immunoreactivity for p53 to be considered as truly mutant/aberrant and further studies, including paired p53 molecular analysis, may be needed to clarify this issue. However, we do recognized that implementing threshold requirements may be challenging in cases lacking full-thickness CIS (i.e., pagetoid, undermining CIS patterns). We also acknowledge the limitation of potential inhomogeneity in this cohort, as immunostains were performed in the respective contributors’ institutions, and NGS was performed at 2 different institutions, using somewhat different approaches.
In summary, in this study we have shown that a null phenotype staining pattern in urothelial CIS (complete loss of staining in the lesional cells) for p53 is indeed aberrant, supported by the NGS analysis demonstrating inactivating TP53 mutations in essentially all evaluated cases. The vast majority of these mutations were nonsense or frameshift mutations, which have been reported with a null phenotype p53 staining in ovarian serous carcinomas33. In addition, the NGS results suggest that a subset of CIS lesions harbor multiple TP53 mutations. Although temporal distinct CIS lesions from a given patient had at least one shared TP53 mutation, some had intratumoral heterogeneity and/or exhibited a clonal evolution. A null phenotype aberrant p53 immunostaining pattern is not currently well-recognized in the genitourinary pathology literature, and its recognition should prompt a consensus reporting of p53 immunostain results (“wild type” versus “mutant”).
References
Levine, A. J., Momand, J. & Finlay, C. A. The p53 tumour suppressor gene. Nature 351, 453–456 (1991).
Iggo, R., Gatter, K., Bartek, J., Lane, D. & Harris, A. L. Increased expression of mutant forms of p53 oncogene in primary lung cancer. Lancet 335, 675–679 (1990).
Lohmann, D., Ruhri, C., Schmitt, M., Graeff, H. & Höfler, H. Accumulation of p53 protein as an indicator for p53 gene mutation in breast cancer. Occurrence of false-positives and false-negatives. Diagn. Mol. Pathol. 2, 36–41 (1993).
Sidransky, D. et al. Identification of p53 gene mutations in bladder cancers and urine samples. Science 252, 706–709 (1991).
Esrig, D. et al. p53 nuclear protein accumulation correlates with mutations in the p53 gene, tumor grade, and stage in bladder cancer. Am. J. Pathol. 143, 1389–1397 (1993).
Kelsey, K. T. et al. A population-based study of immunohistochemical detection of p53 alteration in bladder cancer. Br. J. Cancer 90, 1572–1576 (2004).
Kuczyk, M. A. et al. p53 overexpression as a prognostic factor for advanced stage bladder cancer. Eur. J. Cancer 31A, 2243–2247 (1995).
Serth, J. et al. p53 immunohistochemistry as an independent prognostic factor for superficial transitional cell carcinoma of the bladder. Br. J. Cancer 71, 201–205 (1995).
Amin, M. B., Trpkov, K., Lopez-Beltran, A. & Grignon, D., Members of the ISUP Immunohistochemistry in Diagnostic Urologic Pathology Group. Best practices recommendations in the application of immunohistochemistry in the bladder lesions: report from the International Society of Urologic Pathology consensus conference. Am. J. Surg. Pathol. 38, e20–e34 (2014).
Köbel, M. et al. Interpretation of P53 immunohistochemistry in endometrial carcinomas: toward increased reproducibility. Int. J. Gynecol. Pathol. 38, S123–S131 (2019).
McCluggage, W. G., Soslow, R. A. & Gilks, C. B. Patterns of p53 immunoreactivity in endometrial carcinomas: ‘all or nothing’ staining is of importance. Histopathology 59, 786–788 (2011).
Hodgson, A., Xu, B. & Downes, M. R. p53 immunohistochemistry in high-grade urothelial carcinoma of the bladder is prognostically significant. Histopathology 71, 296–304 (2017).
Neal, D. J., Amin, M. B. & Smith, S. C. CK20 versus AMACR and p53 immunostains in evaluation of Urothelial Carcinoma in Situ and Reactive Atypia. Diagn. Pathol. 15, 61 (2020).
Nguyen, J. K., Przybycin, C. G., McKenney, J. K. & Magi-Galluzzi, C. Immunohistochemical staining patterns of Ki-67 and p53 in florid reactive urothelial atypia and urothelial carcinoma in situ demonstrate significant overlap. Hum. Pathol. 98, 81–88 (2020).
Sangoi, A. R., Chan, E., Stohr, B. A. & Kunju, L. P. Invasive plasmacytoid urothelial carcinoma: a comparative study of E-cadherin and P120 catenin. Hum. Pathol. 102, 54–59 (2020).
McKenney, J. K., Gomez, J. A., Desai, S., Lee, M. W. & Amin, M. B. Morphologic expressions of urothelial carcinoma in situ: a detailed evaluation of its histologic patterns with emphasis on carcinoma in situ with microinvasion. Am. J. Surg. Pathol. 25, 356–362 (2001).
Chan, E., Garg, K. & Stohr, B. A. Integrated immunohistochemical and molecular analysis improves diagnosis of high-grade carcinoma in the urinary bladder of patients with prior radiation therapy for prostate cancer. Mod. Pathol. 33, 1802–1810 (2020).
Arias-Stella, J. A. 3rd, Shah, A. B., Gupta, N. S. & Williamson, S. R. CK20 and p53 immunohistochemical staining patterns in urinary bladder specimens with Equivocal Atypia. Arch. Pathol. Lab. Med. 142, 64–69 (2018).
Aron, M. et al. Utility of a triple antibody cocktail intraurothelial neoplasm-3 (IUN-3-CK20/CD44s/p53) and α-methylacyl-CoA racemase (AMACR) in the distinction of urothelial carcinoma in situ (CIS) and reactive urothelial atypia. Am. J. Surg. Pathol. 37, 1815–1823 (2013).
Lawless, M. E., Tretiakova, M. S., True, L. D. & Vakar-Lopez, F. Flat Urothelial Lesions with Atypia: interobserver concordance and added value of immunohistochemical profiling. Appl. Immunohistochem. Mol. Morphol. 26, 180–185 (2018).
Mallofré, C., Castillo, M., Morente, V. & Solé, M. Immunohistochemical expression of CK20, p53, and Ki-67 as objective markers of urothelial dysplasia. Mod. Pathol. 16, 187–191 (2003).
McKenney, J. K., Desai, S., Cohen, C. & Amin, M. B. Discriminatory immunohistochemical staining of urothelial carcinoma in situ and non-neoplastic urothelium: an analysis of cytokeratin 20, p53, and CD44 antigens. Am. J. Surg. Pathol. 25, 1074–1078 (2001).
Oliva, E. et al. Immunohistochemistry as an adjunct in the differential diagnosis of radiation-induced atypia versus urothelial carcinoma in situ of the bladder: a study of 45 cases. Hum. Pathol. 44, 860–866 (2013).
Pederzoli, F. et al. Diagnosis of urothelial carcinoma in situ using blue light cystoscopy and the utility of immunohistochemistry in blue light-positive lesions diagnosed as atypical. Hum. Pathol. 90, 1–7 (2019).
Sun, W., Zhang, P. L. & Herrera, G. A. p53 protein and Ki-67 overexpression in urothelial dysplasia of bladder. Appl. Immunohistochem. Mol. Morphol. 10, 327–331 (2002).
Yildiz, I. Z. et al. Utility of a dual immunostain cocktail comprising of p53 and CK20 to aid in the diagnosis of non-neoplastic and neoplastic bladder biopsies. Diagn. Pathol. 4, 35 (2009).
Köbel, M. et al. The biological and clinical value of p53 expression in pelvic high-grade serous carcinomas. J. Pathol. 222, 191–198 (2010).
Hartmann, A. et al. Occurrence of chromosome 9 and p53 alterations in multifocal dysplasia and carcinoma in situ of human urinary bladder. Cancer Res. 62, 809–818 (2002).
Sarkis, A. S. et al. Association of P53 nuclear overexpression and tumor progression in carcinoma in situ of the bladder. J. Urol. 152, 388–392 (1994).
Schmitz-Dräger, B. J. et al. P53 accumulation in precursor lesions and early stages of bladder cancer. World J. Urol. 12, 79–83 (1994).
Akhtar, M. et al. Urothelial Carcinoma In Situ (CIS): new insights. Adv. Anat. Pathol. 26, 313–319 (2019).
Amin, M. B. et al. The Genitourinary Pathology Society update on classification and grading of flat and papillary urothelial neoplasia with new reporting recommendations and approach to lesions with mixed and early patterns of Neoplasia. Adv. Anat. Pathol. 28, 179–195 (2021).
Schultheis, A. M. et al. TP53 mutational spectrum in Endometrioid and Serous Endometrial Cancers. Int J. Gynecol Pathol. 35, 289–300 (2016).
Author information
Authors and Affiliations
Contributions
All authors contributed to study design, data analysis, and manuscript review.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
IRB HUM 00042749.
Competing interests
M.S.H. – Consultant and Janssen Pharmaceuticals. All other authors have nothing to report.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sangoi, A.R., Chan, E., Abdulfatah, E. et al. p53 null phenotype is a “positive result” in urothelial carcinoma in situ. Mod Pathol 35, 1287–1292 (2022). https://doi.org/10.1038/s41379-022-01062-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-022-01062-2