Abstract
Mismatch repair protein immunohistochemistry is a widely used method for detecting patients at risk for Lynch syndrome. Recent data suggest that a two-antibody panel approach using PMS2 and MSH6 is an effective screening protocol for colorectal carcinoma, but there are limited data concerning this approach for extraintestinal tumors. The purpose of this study was to review the utility of a two-antibody panel approach in colorectal carcinoma and extraintestinal tumors. We evaluated mismatch repair protein expression in two cohorts: (1) a retrospective analysis of intestinal and extraintestinal tumors (n=334) tested for mismatch repair protein immunohistochemistry and (2) a prospectively accrued series of intestinal, gynecologic tract, and skin sebaceous neoplasms (n=98). A total of 432 cases were analyzed, including 323 colorectal, 50 gynecologic tract, 49 skin sebaceous, and 10 other neoplasms. Overall, 102/432 tumors (24%) demonstrated loss of at least one mismatch repair protein. Concurrent loss of MLH1 and PMS2 was the most common pattern of abnormal expression (50/432, 12%) followed by concurrent loss of MSH2 and MSH6 (33/432, 8%). Of 55 cases with abnormal PMS2 expression, 5 (9%) demonstrated isolated loss of PMS2 expression. Of 47 cases with abnormal MSH6 expression, 14 (30%) demonstrated isolated loss of MSH6 expression. Isolated loss of MLH1 or MSH2 was not observed. Colorectal carcinomas more frequently demonstrated abnormal expression of PMS2 (39/59, 66%). Skin sebaceous neoplasms more frequently demonstrated abnormal expression of MSH6 (18/24, 75%, respectively). A total of 65 tumors with abnormal mismatch repair protein expression were tested for microsatellite instability (MSI): 47 (72%) MSI high, 9 (14%) MSI low, and 9 (14%) microsatellite stable (MSS). Abnormal MSH6 expression accounted for 14/18 (78%) cases that were MSS or MSI low. Our findings confirm the utility of a two-antibody approach using PMS2 and MSH6 in colorectal carcinoma and indicate that this approach is effective in extraintestinal neoplasms associated with Lynch syndrome.
Similar content being viewed by others
Main
In the mid-1960s, Henry Lynch described a family from Nebraska with an unusually high prevalence of early colorectal carcinoma characterized by an absence of polyposis,1 now known as hereditary nonpolyposis colorectal cancer or Lynch syndrome. Currently, Lynch syndrome is the most commonly diagnosed form of hereditary colorectal carcinoma accounting for 3–5% of all colorectal carcinomas. Germline mutations in four DNA mismatch repair genes are thought to give rise to Lynch syndrome: MLH1 (40–45% of cases), MSH2 (40–45%), MSH6 (5–10%), and PMS2 (<5%).2, 3, 4, 5, 6, 7, 8, 9 Currently, in ∼5% of cases a germline mutation in a known DNA mismatch repair gene cannot be identified.1 Affected persons with Lynch syndrome are at risk for neoplasia in the colon but also have an elevated risk of developing tumors of the endometrium, ovary, skin, stomach, biliary tract, and upper urinary tract.10
Over the last decade, there has been increasing interest in identifying those patients with Lynch syndrome who present with extraintestinal disease. In women with Lynch syndrome, the incidence of endometrial carcinoma equals or exceeds that of colorectal carcinoma,10, 11 and the lifetime risk for endometrial carcinoma in Lynch syndrome is between 40 and 60% compared with 3% for the general population.11 Identifying patients at risk for Lynch syndrome requires evaluation of endometrial carcinoma for mismatch repair protein abnormalities, and is important as these patients are at risk for synchronous or metachronous Lynch syndrome-related tumors10 and would benefit from close surveillance and genetic counseling.12 Muir–Torre syndrome (MTS) is a clinical variant of Lynch syndrome, and MTS patients typically present with a cutaneous sebaceous neoplasm and have at least one visceral malignancy.13 Increasingly, patients with multiple sebaceous neoplasms or family history concerning MTS are being screened for mismatch repair protein abnormalities.14, 15, 16, 17, 18 Recent literature has shown that in these patients, mismatch repair protein immunohistochemistry is a useful screening tool.19
Historically, microsatellite instability (MSI) testing was considered superior to mismatch repair protein immunohistochemistry in identifying patients with Lynch syndrome.7 This was primarily because of the limitations of MLH1 immunohistochemistry. The sensitivity of MLH1 immunohistochemistry for detecting germline MLH1 mutations is only ∼74% compared with >90% sensitivity for MSI.7 A small subset of MLH1 truncating and nontruncating mutations results in antigenically active functionally inactive protein, leading to spurious results with MLH1 immunohistochemistry.20, 21, 22 Because of the low sensitivity of MLH1 immunohistochemistry alone, PMS2 has been added to the mismatch repair protein immunohistochemistry panel.22 Recent literature suggests that the use of all four antibodies (MLH1, MSH2, MSH6, and PMS2) is as sensitive in predicting germline mismatch repair protein mutations as MSI testing.23, 24, 25 The addition of MSH6 immunohistochemistry also adds sensitivity, as MSH6 germline mutations often yield only low levels of MSI that may not be detected with the microsatellites tested by PCR analysis.26, 27
In their functional state within a cell, MLH1 dimerizes with PMS2 and MSH2 dimerizes with MSH6.22, 24, 28, 29, 30 MLH1 and MSH2 are the obligatory partners for their respective heterodimers. In general, germline mutations in the obligatory partners MLH1 and MSH2 most often will result in degradation of the heterodimer and the consequent proteolytic degradation of their respective secondary partners, PMS2 and MSH6.29 In contrast, germline mutations in the secondary partners MSH6 and PMS2 may not result in proteolytic degradation of its obligatory partner, as the function of the secondary protein may be compensated by other proteins. Based on this concept, a two-antibody panel of PMS2 and MSH6 for screening colorectal carcinoma by mismatch repair protein immunohistochemistry has been proposed.31 In their study, Shia et al31 reviewed 232 cases of colorectal carcinoma and demonstrated no isolated loss of MLH1 or isolated loss of MSH2, suggesting that a two-antibody screen including MSH6 and PMS2 is efficacious for colorectal carcinoma, potentially reducing the cost of mismatch repair protein immunohistochemistry. Similarly, Hall et al32 confirmed these findings in their analysis of 344 cases of colorectal carcinoma. However, the utility of the two-antibody panel has not been well studied in extraintestinal Lynch syndrome-related tumors. Herein, we report the findings of our examination of mismatch repair protein immunohistochemistry to determine the utility of the two-antibody mismatch repair protein immunohistochemistry screening approach in intestinal and extraintestinal tumors with particular attention to gynecologic tract and sebaceous tumors.
Materials and methods
Study Groups
We analyzed two groups of patients who had testing for mismatch repair protein immunohistochemistry with or without concurrent MSI testing. The first group consisted of a retrospective analysis of all intestinal and extraintestinal tumors tested for mismatch repair protein immunohistochemistry within the archives of the Department of Pathology at Stanford University Medical Center from January 2003 through April 2010. The cases were selected for analysis based on at least one of the following: (1) age <50 years, (2) clinical concern for Lynch syndrome meeting requirements of the Bethesda criteria (ie, patient with synchronous or metachronous colorectal carcinoma or other Lynch syndrome-associated tumor regardless of age; colorectal carcinoma diagnosed in one or more first-degree relatives with a Lynch syndrome-related tumor, with one of the cancers being diagnosed at <50 years of age; or colorectal carcinoma diagnosed in two or more first- or second-degree relatives with Lynch syndrome-related tumors, regardless of age), and (3) colorectal tumor morphology associated with abnormal DNA mismatch repair including mucinous, signet-ring, or medullary morphology, increased tumor-infiltrating lymphocytes, and/or peri-tumoral lymphoid aggregates.33 The second group was a prospective analysis that included all intestinal carcinomas, endometrial carcinomas, and skin sebaceous neoplasms accrued from April 2010 through November 2010 and tested for mismatch repair protein immunohistochemistry regardless of patient age or tumor morphology.
Mismatch Repair Protein Immunohistochemistry
Mismatch repair protein immunohistochemistry was performed using the standard streptavidin–biotin–peroxidase procedure. Primary monoclonal antibodies against MLH1 (clone G168-728; BD PharMingen, San Diego, CA, USA; 1:200), MSH2 (clone FE11; Oncogene Research Products, Cambridge, MA, USA; 1:100), MSH6 (clone 44; BD Transduction, San Jose, CA, USA; 1:200), and PMS2 (clone A16-4, BD Biosciences, San Jose, CA, USA; 1:10) were applied to 5-μm-thick formalin-fixed, paraffin-embedded whole tumor sections. The sections were deparaffinized in xylene, and rehydrated through graded alcohols to distilled water before undergoing antigen retrieval by heat treatment in either citrate solution pH 6.0 (MLH1, PMS2, and MSH2) or EDTA solution pH 9.0 (MSH6). An automated detection using the Ventana Benchmark XT Autostainer (Tucson, AZ, USA) was employed. Normal expression was defined as nuclear staining within tumor cells, using infiltrating lymphocytes as positive internal control. Negative protein expression was defined as complete absence of nuclear staining within tumor cells in the face of concurrent positive labeling in internal nonneoplastic tissues. Cases with immunoreactivity in <10% of tumor cells were repeated, and if the findings were confirmed then they were scored as equivocal. Cases lacking any expression in both tumor and nonneoplastic tissues underwent repeat immunohistochemical analysis using the same conditions. For PMS2, cases in the retrospective group lacking any expression in both tumor and nonneoplastic tissue were re-evaluated using an alternate PMS2 antibody (clone MRQ-28; Cell Marque, Rocklin, CA, USA; 1:10 with citrate antigen retrieval). All cases in the prospective group were analyzed with this alternate PMS2 antibody (clone MRQ-28; Cell Marque; 1:10 with citrate antigen retrieval). In addition, for the prospective group, two pathologists independently scored each case using only the MSH6 and PMS2 studies and documented their interpretation and overall impression of whether the two-antibody approach was sufficient for analysis of mismatch repair protein expression for each case.
MSI Polymerase Chain Reaction (PCR)
In the retrospective group, MSI analysis was performed on 117 cases of colorectal carcinomas reviewed before June 2007 using the Bethesda Panel of five microsatellite markers proposed by the National Cancer Institute (NCI): BAT25, BAT26, D2S123, D5S346, and D17S250.34 Thereafter, all colorectal carcinomas, sebaceous neoplasms, and gynecologic tract tumors were analyzed using the ProMega MSI analysis system utilizing a panel of five mononucleotide microsatellite markers (BAT-25, BAT-26, NR-21, NR-24, and MONO-27) and two pentanucleotide repeats (Penta C and Penta D) incorporated into a multiplex fluorescence assay.35 Briefly, the tests were performed on tumor and normal DNA extracted from paraffin-embedded tissue blocks using a DNease Tissue Kit (Qiagen, Valencia, CA, USA); manual microdissection was performed, when required, to exclude overabundance of nonlesional tissue. Paired DNA samples from neoplastic and nonneoplastic samples were genotyped and analyzed by capillary electrophoresis using an ABI 310 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). In accordance with NCI guidelines, MSI at ≥2 loci was defined as MSI high (MSI-H), instability at a single locus was defined as MSI low (MSI-L), and no instability at any of the loci tested was defined as microsatellite stable (MSS).
Statistical Analysis
Fisher's exact test (two tailed), χ2, or Student's t-tests were used to evaluate the correlation of mismatch repair protein immunohistochemistry and clinicopathological characteristics. The significance level was set at P<0.05.
Results
A total of 334 cases were in the retrospective group, including 260 colorectal carcinomas, 42 skin sebaceous neoplasms, 26 gynecologic tract carcinomas, and 6 neoplasms from various sites (stomach, ampulla, and central nervous system). A total of 98 cases were in the prospective group, including 63 colorectal carcinomas, 7 skin sebaceous neoplasms, 24 gynecologic carcinomas, and 4 small intestinal carcinomas. Overall, including both retrospective and prospective analyses, 59 of 323 (18%) colorectal carcinomas demonstrated loss of at least one mismatch repair protein (Table 1). The median age of patients with colorectal carcinomas with abnormal mismatch repair protein expression was 44 years (range 26–80 years). Colorectal carcinomas with abnormal mismatch repair protein expression occurred more often in men (36/59, 61%) than women (23/59, 39%; P=0.04). The most common pattern of loss was concurrent MLH1 and PMS2 (34 cases, 11%) followed by concurrent loss of MSH2 and MSH6 (12 cases, 4%). Five (1.5%) colorectal carcinomas demonstrated isolated loss of PMS2 (Figure 1). Eight (2.5%) colorectal carcinomas displayed isolated loss of MSH6. Importantly, none of the colorectal carcinomas demonstrated isolated loss of MLH1 or isolated loss of MSH2. Of the 59 colorectal carcinomas demonstrating loss of at least one mismatch repair protein by immunohistochemistry, 49 (83%) were evaluated by MSI PCR (Table 2). Of the 10 MSS/MSI-L tumors, 9 had their MSI analysis performed using the Bethesda Panel rather than the Promega MSI analysis system. Concurrent loss of MLH1/PMS2 was most often associated with MSI-H (28/29 cases, 97%) with only 1 case (3%) demonstrating MSI-L. Concurrent loss of MSH2/MSH6 also most often resulted in MSI-H (9/12 cases, 75%) but MSS (2/12 cases, 17%) and MSI-L (1/12 cases, 8%) were also identified. Those cases with isolated loss of MSH6 more often demonstrated MSS (two cases) or MSI-L (three cases), confirming the benefit of MSH6 immunohistochemistry compared with MSI PCR, particularly if performed using the Bethesda Panel of microsatellite markers. All colorectal carcinoma cases with intact expression of all four mismatch repair proteins evaluated by MSI PCR were MSS.
Overall, including both retrospective and prospective analyses, we evaluated 50 gynecologic tract carcinomas, comprising 40 uterine primary carcinomas and 10 ovarian primary carcinomas (Tables 1 and 3). Of 50 gynecologic carcinoma cases, 19 (38%) demonstrated loss of at least one mismatch repair protein. The median age of patients with gynecologic tract neoplasms with abnormal mismatch repair protein expression was 58 years (range 42–70 years). The most common patterns of loss were concurrent loss of MLH1 and PMS2 (10 cases, 20%) and isolated loss of MSH6 (5 cases, 10%) (Figure 2). Concurrent loss of MSH2 and MSH6 was seen in 4 cases (8%). No cases demonstrated isolated loss of PMS2. Importantly, no case demonstrated isolated loss of MLH1 or isolated loss of MSH2. All 19 cases with abnormal mismatch repair protein immunohistochemistry were endometrioid carcinomas, including 17 primary uterine endometrioid carcinomas and 2 primary ovarian endometrioid carcinomas (Table 3). Of the endometrioid carcinomas, tumors with abnormal mismatch repair protein expression were more often grade 3 (12/20, 60%) compared with grade 1 and 2 carcinomas (7/21, 33%), although this did not reach statistical significance (P=0.12). No cases of clear cell (0/7) or serous (0/2) carcinoma demonstrated abnormal mismatch repair protein expression. Of the 19 cases with abnormal mismatch repair protein immunohistochemistry, 8 had microsatellite PCR analysis performed: 5 were MSI-H and four demonstrated no evidence of MSI (Table 3). Of the four MSS tumors, three had abnormalities of MSH6: two cases with isolated loss of MSH6 and one case with concurrent loss of MSH2 and MSH6. All gynecologic tract carcinomas with intact expression of all four mismatch repair proteins evaluated by MSI PCR were MSS.
Of the 49 skin sebaceous tumors included in the retrospective and prospective analyses, most were either sebaceous adenomas or sebaceomas (26/49, 53%), with fewer numbers of sebaceous carcinomas (12 cases, 24%) and atypical sebaceous neoplasms (11 cases, 22%; Table 4). Overall, 24 cases (49%) demonstrated loss of at least one mismatch repair protein. The median age of patients with skin sebaceous neoplasms with abnormal mismatch repair protein expression was 62 years (range 49–91 years). In contrast to colorectal carcinoma, the most common pattern of loss was concurrent loss of MSH2 and MSH6 accounting for 17 (35%) cases. Fewer cases of concurrent loss of MLH1 and PMS2 were identified accounting for 6 (12%) cases. Isolated loss of MSH6 was seen in one (2%) case. No cases demonstrated isolated loss of PMS2. Importantly, no case demonstrated isolated loss of MLH1 or isolated loss of MSH2. Of the 24 cases demonstrating abnormal mismatch repair protein immunohistochemistry, most were sebaceous adenoma/sebaceoma diagnoses (12 cases) with fewer numbers of sebaceous carcinomas (3 cases) and atypical sebaceous neoplasms (9 cases). Of the 24 sebaceous tumors with abnormal mismatch repair protein expression, 7 were submitted for MSI PCR analysis with variable results: 3 MSI-H, 3 MSI-L, and 1 MSS. All MSI-L/MSS cases were sebaceous adenoma/sebaceoma, whereas all MSI-H cases were either atypical sebaceous neoplasms or sebaceous carcinomas. Of the four MSS/MSI-L tumors, three had abnormalities of MSH6: one case with isolated loss of MSH6 and two cases with concurrent loss of MSH2 and MSH6. Cases were also stratified by anatomic location into two categories: head or neck (31 cases) versus trunk or extremities (15 cases). The anatomic location of the tumor for three cases was unknown. Abnormal mismatch repair protein expression was less often identified in sebaceous tumors of the head or neck (10/31, 32%) compared with sebaceous tumors of the trunk or extremities (12/15, 80%; P=0.004). Skin sebaceous neoplasms with abnormal mismatch repair protein expression occurred more often in men (21/24, 88%) than women (3/24, 13%; P=0.17).
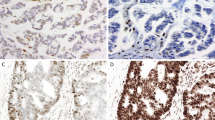
A lack of PMS2 expression in both tumor and nonneoplastic tissue was observed in 13 colorectal carcinomas in our retrospective analysis; none of the gynecologic tract carcinomas, skin sebaceous neoplasms, or colorectal carcinomas in the prospective group exhibited this PMS2 staining pattern. The colorectal carcinomas exhibiting a lack of PMS2 immunoreactivity were re-evaluated using a different PMS2 antibody clone (MRQ-28). Using this alternate PMS2 antibody, eight cases exhibited intact PMS2 expression and five cases exhibited loss of PMS2 expression. We also encountered equivocal MSH6 staining (defined as <10% immunoreactivity in the neoplastic cells) in both the retrospective and prospective groups; however, equivocal MSH6 staining was only seen in the extraintestinal tumors (skin sebaceous neoplasms, two cases; gynecologic tract carcinomas, two cases; and small bowel carcinoma, one case) (Figure 3). All five cases with equivocal MSH6 staining exhibited intact expression of MSH2. In our prospective review using the two-antibody (PMS2 and MSH6) approach, equivocal MSH6 staining was identified in 4/98 (4%) cases and was the only deficiency noted with the two-antibody panel.
Equivocal immunoreactivity with MSH6 identified in a skin sebaceous adenoma (a, × 200) that demonstrates immunoreactivity in lesional cells present at the periphery of the lesion and that account for <10% of the cells. Equivocal immunoreactivity with MSH6 in a primary uterine endometrioid carcinoma (b, × 400) in which most of the tumor cells lacked MSH6 expression with areas demonstrating weak nuclear MSH6 staining comprising <10% of tumor cells.
Discussion
Based on the heterodimer concept of mismatch repair proteins, we investigated a two-antibody immunohistochemical screening approach using PMS2 and MSH6 in colorectal carcinomas and extraintestinal Lynch syndrome-related neoplasms. In our analysis, PMS2 immunohistochemical analysis detected all cases with either MLH1 or PMS2 loss of expression. Similarly, the MSH6 immunohistochemical analysis detected all cases with either MSH2 or MSH6 loss of expression. Thus, no study cases exhibited isolated loss of expression of MLH1 or isolated loss of expression of MSH2. Our results confirm the utility of the two-antibody panel immunohistochemical screening approach in colorectal carcinoma as described by Shia et al,31 and further indicate that the two-antibody approach is applicable to extraintestinal sites, particularly skin sebaceous neoplasms and gynecologic tract carcinomas.
Numerous reports have evaluated mismatch repair protein immunohistochemistry in skin sebaceous neoplasms, although most studies have employed only MLH1 and MSH2 in their immunohistochemical analysis.14, 18, 36, 37, 38 To our knowledge, only one study has analyzed the expression of all four mismatch repair proteins in skin sebaceous neoplasms (Table 5).19 Orta et al19 analyzed 27 patients with one or more sebaceous neoplasms and showed abnormal mismatch repair protein expression in 12 (44%) patients (Table 4). Our analysis revealed a somewhat higher proportion (49%) of skin sebaceous neoplasms with abnormal mismatch repair protein expression, likely because patient selection for inclusion in our retrospective analysis was primarily based on clinical concern for MTS after evaluation of clinical characteristics and family history. Importantly, in their analysis, Orta et al19 also demonstrated that loss of expression of MLH1 and MSH2 was always coupled with concurrent loss of expression of PMS2 and MSH6, respectively (Table 4). Our results also demonstrate that abnormal mismatch repair protein expression is much more frequent in skin sebaceous tumors located on the trunk or extremities compared with head or neck and in men compared with women, similar to other reports in the literature.14, 19
Gynecologic tract carcinomas have been more frequently studied with immunohistochemical antibodies for all four mismatch repair proteins than skin sebaceous tumors (Table 5). Modica et al39 analyzed 85 endometrial carcinoma patients with interpretable immunohistochemistry and identified 48 cases (56%) with loss of expression of at least one mismatch repair protein. Notably, one endometrial carcinoma case demonstrated isolated loss of MSH2 with intact expression of MSH6. Another study evaluated 71 patients with uterine carcinomas with clinical and/or morphologic characteristics concerning for Lynch syndrome and found 32 cases (45%) with loss of at least one mismatch repair protein.40 In their analysis, Garg et al40 identified 19 cases with MLH1/PMS2 loss, 9 cases with concurrent MSH2 and MSH6 loss, and 4 cases with isolated loss of MSH6. In a prospective analysis of 140 uterine carcinomas by Backes et al,41 30 patients had loss of at least one mismatch repair protein (21%), with 4 cases with concurrent MSH2 and MSH6 loss, 24 cases with concurrent MLH1 and PMS2 loss, and 2 cases of isolated MSH6 loss.41 Both Garg et al40 and Backes et al41 identified no gynecologic tract carcinomas with isolated loss of MSH2 or MLH1 similar to our findings.
Our study was limited to only 10 extracolonic cases outside of the gynecologic tract and skin, and very little data are available in the literature regarding mismatch repair protein immunohistochemistry in these sites.42 Of the 10 cases in our series, only one small bowel adenocarcinoma demonstrated equivocal MSH6 immunoreactivity, with the remainder demonstrating intact mismatch repair protein expression. Further analysis of these other potential Lynch syndrome-related tumors should be performed before any definite recommendations can be made.
Difficulties in interpretation of mismatch repair protein immunohistochemistry did occur in our analysis and mostly relate to staining quality that may render interpretation of the stains difficult.7 First, MSH6 staining is frequently patchy and weak in gynecologic tract carcinomas and skin sebaceous neoplasms, making confident interpretation difficult. Focal, weak immunoreactivity with MSH6 has been previously reported in colorectal carcinomas.43 In addition, recently, partial intact MSH6 expression in colorectal carcinoma has been demonstrated to occur after neoadjuvant therapy or rarely in MLH1- and/or PMS2-deficient colorectal carcinomas.44, 45 In our analysis, we identified a small number of gynecologic tract and skin sebaceous tumors with focal MSH6 immunoreactivity; none of these patients had received chemotherapy or had concurrent loss of immunohistochemical expression of any other mismatch repair proteins. Interestingly, in our analysis, focal MSH6 immunoreactivity was limited to extracolonic tumors with no cases of colorectal carcinoma demonstrating this pattern of immunoreactivity. Second, a minor subset of colorectal carcinomas in our retrospective analysis demonstrated lack of PMS2 immunoreactivity in both the tumor and normal tissues. With repeat analysis with a different PMS2 antibody clone (MRQ-28), we were able to reclassify PMS2 expression as intact or lost in these 13 colorectal carcinoma cases. Improved immunohistochemical procedures also likely had an impact on our analysis with PMS2 as most cases that lacked immunoreactivity in both normal and tumor tissues were cases from the early period of our retrospective analysis. Finally, the recognition of scattered lymphocyte reactivity of mismatch repair proteins within a tumor should be emphasized in assessing tumor cell reactivity. In our analysis, a small subset of cases with loss of mismatch repair protein expression were initially misinterpreted as tumor exhibiting intact mismatch repair protein expression by pathologists without knowledge of the potential pitfall of tumor-infiltrating lymphocytes. Such interpretative errors can be minimized by limiting the analysis of these studies to pathologists with sufficient exposure in assessing mismatch repair protein immunohistochemistry.
In clinical practice, we propose that the two-antibody panel including PMS2 and MSH6 can function as an initial screen in evaluation of Lynch syndrome-related tumors for abnormal mismatch repair protein immunohistochemistry. In all of the problem cases, particularly those cases with equivocal MSH6 results identified in our analysis, the inclusion of MLH1 and MSH2 analysis would not have changed the eventual interpretation of the immunohistochemistry results. Thus, an initial screen using two-antibody (PMS2 and MSH6) panel is equally as effective as the conventional four-antibody panel in screening for patients who harbor tumors with mismatch repair protein abnormalities and who should be further evaluated for the possibility of Lynch syndrome. However, after the initial two-antibody (PMS2 and MSH6) screen, any case exhibiting abnormal or equivocal mismatch repair protein expression should be evaluated with MLH1 and MSH2, as knowledge of the specific mismatch repair protein deficiency is extremely useful in helping to direct gene sequencing efforts to identify the disease-causing mutation in patients suspected of having Lynch syndrome. Importantly, immunohistochemical staining for mismatch repair proteins is complementary to MSI PCR studies. In general, immunohistochemistry is routinely available in general pathology laboratories and is less expensive compared with PCR detection of MSI.46 In addition, mismatch repair immunohistochemistry can identify patients with low levels of MSI that may be missed by PCR analysis, particularly those patients with mutations in MSH6.25, 26 Our analysis confirms the utility of MSH6 immunohistochemistry when screening for Lynch syndrome, as abnormalities of MSH6 expression accounted for most (78%) of the cases that were MSS or MSI-L by PCR analysis. However, MSI testing by PCR can potentially identify patients with defective DNA mismatch repair but intact immunohistochemical staining as a result of nontruncating missense alterations or defects in genes other than the four mismatch repair proteins routinely tested for by immunohistochemistry. Thus, in those patients with clinical concern for Lynch syndrome meeting requirements of the Bethesda criteria, both mismatch repair protein immunohistochemistry and MSI PCR should be performed.
In conclusion, a two-antibody panel including PMS2 and MSH6 is a cost-effective screening approach for identifying mismatch repair protein abnormalities in colorectal carcinoma, gynecologic tract carcinomas, and skin sebaceous neoplasms in patients suspected of having Lynch syndrome. Our analysis indicates that the two-antibody panel approach is equally effective as the conventional four-antibody panel in screening for patients who harbor tumors with mismatch repair protein abnormalities. One limitation of the two-antibody approach is the occurrence of equivocal MSH6 immunoreactivity that is more often observed in extracolonic Lynch syndrome-associated neoplasms. Finally, mismatch repair protein immunohistochemistry interpretative errors can be minimized by limiting the analysis of these studies to pathologists with sufficient exposure and knowledge of the potential interpretative pitfalls.
References
Lynch HT, Lynch JF . Lynch syndrome: history and current status. Dis Markers 2004;20:181–198.
Fishel R, Lescoe MK, Rao MR, et al. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell 1993;75:1027–1038.
Papadopoulos N, Nicolaides NC, Wei YF, et al. Mutation of a mutL homolog in hereditary colon cancer. Science 1994;263:1625–1629.
Leach FS, Nicolaides NC, Papadopoulos N, et al. Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell 1993;75:1215–1225.
Bronner CE, Baker SM, Morrison PT, et al. Mutation in the DNA mismatch repair gene homologue hMLH1 is associated with hereditary non-polyposis colon cancer. Nature 1994;368:258–261.
Burgart LJ . Testing for defective DNA mismatch repair in colorectal carcinoma: a practical guide. Arch Pathol Lab Med 2005;129:1385–1389.
Shia J . Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part I. The utility of immunohistochemistry. J Mol Diagn 2008;10:293–300.
Peltomaki P, Vasen H . Mutations associated with HNPCC predisposition--update of ICG-HNPCC/INSiGHT mutation database. Dis Markers 2004;20:269–276.
Hendriks YM, Jagmohan-Changur S, van der Klift HM, et al. Heterozygous mutations in PMS2 cause hereditary nonpolyposis colorectal carcinoma (Lynch syndrome). Gastroenterology 2006;130:312–322.
Aarnio M, Sankila R, Pukkala E, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer 1999;81:214–218.
Lu KH, Dinh M, Kohlmann W, et al. Gynecologic cancer as a “sentinel cancer” for women with hereditary nonpolyposis colorectal cancer syndrome. Obstet Gynecol 2005;105:569–574.
de Jong AE, Hendriks YM, Kleibeuker JH, et al. Decrease in mortality in Lynch syndrome families because of surveillance. Gastroenterology 2006;130:665–671.
South CD, Hampel H, Comeras I, et al. The frequency of Muir-Torre syndrome among Lynch syndrome families. J Natl Cancer Inst 2008;100:277–281.
Cesinaro AM, Ubiali A, Sighinolfi P, et al. Mismatch repair proteins expression and microsatellite instability in skin lesions with sebaceous differentiation: a study in different clinical subgroups with and without extracutaneous cancer. Am J Dermatopathol 2007;29:351–358.
Entius MM, Keller JJ, Drillenburg P, et al. Microsatellite instability and expression of hMLH-1 and hMSH-2 in sebaceous gland carcinomas as markers for Muir-Torre syndrome. Clin Cancer Res 2000;6:1784–1789.
Mangold E, Pagenstecher C, Leister M, et al. A genotype-phenotype correlation in HNPCC: strong predominance of msh2 mutations in 41 patients with Muir-Torre syndrome. J Med Genet 2004;41:567–572.
Kruse R, Rutten A, Schweiger N, et al. Frequency of microsatellite instability in unselected sebaceous gland neoplasias and hyperplasias. J Invest Dermatol 2003;120:858–864.
Mathiak M, Rutten A, Mangold E, et al. Loss of DNA mismatch repair proteins in skin tumors from patients with Muir-Torre syndrome and MSH2 or MLH1 germline mutations: establishment of immunohistochemical analysis as a screening test. Am J Surg Pathol 2002;26:338–343.
Orta L, Klimstra DS, Qin J, et al. Towards identification of hereditary DNA mismatch repair deficiency: sebaceous neoplasm warrants routine immunohistochemical screening regardless of patient's age or other clinical characteristics. Am J Surg Pathol 2009;33:934–944.
Salahshor S, Koelble K, Rubio C, et al. Microsatellite instability and hMLH1 and hMSH2 expression analysis in familial and sporadic colorectal cancer. Lab Invest 2001;81:535–541.
Mangold E, Pagenstecher C, Friedl W, et al. Tumours from MSH2 mutation carriers show loss of MSH2 expression but many tumours from MLH1 mutation carriers exhibit weak positive MLH1 staining. J Pathol 2005;207:385–395.
de Jong AE, van Puijenbroek M, Hendriks Y, et al. Microsatellite instability, immunohistochemistry, and additional PMS2 staining in suspected hereditary nonpolyposis colorectal cancer. Clin Cancer Res 2004;10:972–980.
Hampel H, Frankel WL, Martin E, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med 2005;352:1851–1860.
Southey MC, Jenkins MA, Mead L, et al. Use of molecular tumor characteristics to prioritize mismatch repair gene testing in early-onset colorectal cancer. J Clin Oncol 2005;23:6524–6532.
Lagerstedt Robinson K, Liu T, Vandrovcova J, et al. Lynch syndrome (hereditary nonpolyposis colorectal cancer) diagnostics. J Natl Cancer Inst 2007;99:291–299.
Berends MJ, Wu Y, Sijmons RH, et al. Molecular and clinical characteristics of MSH6 variants: an analysis of 25 index carriers of a germline variant. Am J Hum Genet 2002;70:26–37.
Buttin BM, Powell MA, Mutch DG, et al. Penetrance and expressivity of MSH6 germline mutations in seven kindreds not ascertained by family history. Am J Hum Genet 2004;74:1262–1269.
Umar A, Koi M, Risinger JI, et al. Correction of hypermutability, N-methyl-N’-nitro-N-nitrosoguanidine resistance, and defective DNA mismatch repair by introducing chromosome 2 into human tumor cells with mutations in MSH2 and MSH6. Cancer Res 1997;57:3949–3955.
Boland CR, Koi M, Chang DK, et al. The biochemical basis of microsatellite instability and abnormal immunohistochemistry and clinical behavior in Lynch syndrome: from bench to bedside. Fam Cancer 2008;7:41–52.
Acharya S, Wilson T, Gradia S, et al. hMSH2 forms specific mispair-binding complexes with hMSH3 and hMSH6. Proc Natl Acad Sci USA 1996;93:13629–13634.
Shia J, Tang LH, Vakiani E, et al. Immunohistochemistry as first-line screening for detecting colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome: a 2-antibody panel may be as predictive as a 4-antibody panel. Am J Surg Pathol 2009;33:1639–1645.
Hall G, Clarkson A, Shi A, et al. Immunohistochemistry for PMS2 and MSH6 alone can replace a four antibody panel for mismatch repair deficiency screening in colorectal adenocarcinoma. Pathology 2010;42:409–413.
Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 2004;96:261–268.
Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 1998;58:5248–5257.
Suraweera N, Duval A, Reperant M, et al. Evaluation of tumor microsatellite instability using five quasimonomorphic mononucleotide repeats and pentaplex PCR. Gastroenterology 2002;123:1804–1811.
Ponti G, Losi L, Di Gregorio C, et al. Identification of Muir-Torre syndrome among patients with sebaceous tumors and keratoacanthomas: role of clinical features, microsatellite instability, and immunohistochemistry. Cancer 2005;103:1018–1025.
Ponti G, Losi L, Pedroni M, et al. Value of MLH1 and MSH2 mutations in the appearance of Muir-Torre syndrome phenotype in HNPCC patients presenting sebaceous gland tumors or keratoacanthomas. J Invest Dermatol 2006;126:2302–2307.
Singh RS, Grayson W, Redston M, et al. Site and tumor type predicts DNA mismatch repair status in cutaneous sebaceous neoplasia. Am J Surg Pathol 2008;32:936–942.
Modica I, Soslow RA, Black D, et al. Utility of immunohistochemistry in predicting microsatellite instability in endometrial carcinoma. Am J Surg Pathol 2007;31:744–751.
Garg K, Leitao Jr MM, Kauff ND, et al. Selection of endometrial carcinomas for DNA mismatch repair protein immunohistochemistry using patient age and tumor morphology enhances detection of mismatch repair abnormalities. Am J Surg Pathol 2009;33:925–933.
Backes FJ, Leon ME, Ivanov I, et al. Prospective evaluation of DNA mismatch repair protein expression in primary endometrial cancer. Gynecol Oncol 2009;114:486–490.
Agaram NP, Shia J, Tang LH, et al. DNA mismatch repair deficiency in ampullary carcinoma: a morphologic and immunohistochemical study of 54 cases. Am J Clin Pathol 2010;133:772–780.
Shia J, Klimstra DS, Nafa K, et al. Value of immunohistochemical detection of DNA mismatch repair proteins in predicting germline mutation in hereditary colorectal neoplasms. Am J Surg Pathol 2005;29:96–104.
Guo M, Klimstra DS, Tang LH, et al. Immunohistochemistry for DNA mismatch repair proteins in colorectal carcinoma: what does scanty staining for MSH6 mean? Mod Pathol 2010;23 (Suppl 1):433A.
Bao F, Rennert H, Yantiss RK . Neoadjuvant therapy induces loss of MSH6 expression in colorectal carcinoma. Mod Pathol 2010;23 (Suppl 1):136A.
Debniak T, Kurzawski G, Gorski B, et al. Value of pedigree/clinical data, immunohistochemistry and microsatellite instability analyses in reducing the cost of determining hMLH1 and hMSH2 gene mutations in patients with colorectal cancer. Eur J Cancer 2000;36:49–54.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Mojtahed, A., Schrijver, I., Ford, J. et al. A two-antibody mismatch repair protein immunohistochemistry screening approach for colorectal carcinomas, skin sebaceous tumors, and gynecologic tract carcinomas. Mod Pathol 24, 1004–1014 (2011). https://doi.org/10.1038/modpathol.2011.55
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2011.55
Keywords
This article is cited by
-
Muir–Torre Syndrome: A Cutaneous Finding Amidst Broader Malignancies
American Journal of Clinical Dermatology (2023)
-
Comparison of universal screening in major lynch-associated tumors: a systematic review of literature
Familial Cancer (2022)
-
MSI testing
Der Pathologe (2021)
-
Mismatch repair protein loss in breast cancer: clinicopathological associations in a large British Columbia cohort
Breast Cancer Research and Treatment (2020)
-
Two-stain immunohistochemical screening for Lynch syndrome in colorectal cancer may fail to detect mismatch repair deficiency
Modern Pathology (2018)