Abstract
The stroma in ovarian clear cell carcinoma often shows alternate mucoid and hyalinized change. The hyalinized stroma is recognized to be an aberrant deposition of basement membrane material produced by tumor cells. The mucoid stroma, however, has drawn far less attention, and its significance remains unclear. We examined 60 ovarian clear cell carcinomas for the distribution and nature of the mucoid stroma. For comparison, 125 other surface epithelial ovarian tumors were examined. Twenty-nine of 60 (48%) clear cell carcinomas showed a mucoid stroma, either focally (21 cases) or diffusely (8 cases). The mucoid stroma in clear cell carcinomas was distinct from that in other surface epithelial tumors as follows: it showed a compact spherule-like appearance, commonly occupying the cores of small papillae. It also exhibited a cribriform pattern, resembling that of adenoid cystic carcinoma. It was rarely associated with stromal cells, despite the presence of abundant glycosaminoglycan including hyaluronan. Alternatively, it was strongly associated with hyalinized stroma. Among 40 clear cell carcinomas that had at least one type of stroma, 26 (65%) had both, either concomitantly or separately. The mucoid stroma tended to attenuate if the hyalinized stroma developed. In vitro, a clear cell carcinoma cell line, HAC-2, formed a spherule-like structure containing hyaluronan in the center, and a significant amount of hyaluronan was detected by latex agglutination immunoturbidimetry, indicating that HAC-2 itself has the potential to produce hyaluronan. All of these facts indicate that the spherule-like mucoid stroma and hyalinized stroma represent different phases of the stromal remodeling process, which is promoted by the deposition of different extracellular matrices produced by clear cell carcinoma cells. The spherule-like mucoid stroma and hyalinized stroma are considered complementary diagnostic signatures of ovarian clear cell carcinoma.
Similar content being viewed by others
Main
During cancer development, cancer cells remodel the local host tissue into their own stroma. Among ovarian cancers, clear cell carcinoma is unique for its stroma, as well as its distinct cell type. The most recognized stromal change is hyalinization.1, 2 The deposition of basement membrane material, including laminin-5 and type IV collagen, is responsible for hyalinization.3 Although less recognized, basophilic mucoid stroma is often encountered in ovarian clear cell carcinomas. It usually shows a compact spherule-like appearance and may adjoin the eosinophilic hyalinized stroma, producing a striking dual-colored contrast. This spherule-like mucoid stroma, however, has drawn far less attention than hyalinized stroma, and its exact distribution or source remains unclear. In this study, we investigated 60 ovarian clear cell carcinomas to elucidate the nature of the spherule-like mucoid stroma.
Materials and methods
Surgical Specimens
We investigated 60 clear cell carcinomas of the ovary that had been surgically resected from patients. The patients' ages ranged from 30 to 77 years (mean, 53 years). Tumor size varied from 4 to 25 cm (median, 11 cm). Forty-two patients were stage I, 5 were stage II, and 13 were stage III according to the criteria of the International Federation of Gynecology and Obstetrics (FIGO).4 For a comparative study, 40 serous adenocarcinomas, 40 endometrioid adenocarcinomas, 20 mucinous adenocarcinomas, and 25 borderline tumors (3 clear, 7 serous, and 15 mucinous borderline tumors) of the ovary were investigated. All of the materials were fixed in formalin and embedded in paraffin. A total of 2–5 slides were examined for each case.
Mucin Histochemistry and Immunohistochemistry
Serial sections were stained with hematoxylin–eosin, alcian blue pH 2.5, alcian blue pH 2.5 with hyaluronidase digestion, and periodic acid-Schiff with diastase digestion (d-PAS), and were immunostained with anti-laminin γ2 chain antibody.
For immunohistochemistry, a mouse monoclonal antibody raised against human laminin γ2 chain, a subunit characteristic of laminin-5 (D4B5, dilution 1/100; Chemicon International, Temecula, CA, USA) was used. For antigen retrieval, paraffin-embedded sections were digested with 0.1% protease type XXVII (Sigma, St Louis, MO, USA) for 10 min at 37°C. The slides were exposed to 10% non-immunized rabbit serum in phosphate-buffered saline for 10 min to block non-specific binding of the antibody. Then, the slides were incubated with anti-laminin-5 γ2 chain antibody at 4°C overnight. The immunohistochemical reactions were visualized using a Histofine SAB-PO kit (Nichirei, Tokyo, Japan).
Cell Culture and Heterotransplantation
An ovarian clear cell carcinoma cell line, HAC-2, was seeded on a Type I collagen-coated six-well dish, and cultured in RPMI1640 supplemented with 10% fetal bovine serum in a humidified 5% CO2 atmosphere at 37°C. HAC-2 was generously provided by Dr M Nishida (Kasumigaura Medical Center, Tsuchiura, Japan). As a negative control, three wells containing the medium alone were accompanied by the above wells. After HAC-2 was cultured to confluence, the cells in three wells were gently scraped to make a cell block. From the other three wells, the medium was removed, then the cells and subcellular matrix remaining on the well surface were collected by scraping in 1 ml phosphate-buffered saline per well. The cell suspension of each well was centrifuged at 3000 r.p.m. for 3 min, and the supernatant was obtained for measurement of hyaluronan by latex agglutination immunoturbidimetry (SRL, Tokyo, Japan).
Approximately 2 × 107 cells were suspended in 0.2 ml of RPMI1640 containing 10% fetal bovine serum and injected into the abdominal cavity of 4-week-old female nude mice (BALB/c-Nu/Nu). About 40–60 days after injection, the tumors were obtained, fixed in 10% formalin and embedded in paraffin. This study was carried out in accordance with the Guide for Animal Experimentation, Yamagata University School of Medicine and Japanese Governmental Law.
Results
Surgical Specimens
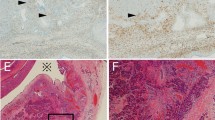
On hematoxylin and eosin sections, 29 of 60 (48%) clear cell carcinomas had basophilic mucoid stroma of compact spherule-like appearance, focally (21 cases) or diffusely (8 cases). There was no significant correlation between this feature and patient age, tumor size or FIGO stage. The spherule-like mucoid stroma was found either in the area showing a papillary architecture (25 cases) or in the area showing a solid architecture (4 cases). In the former, basophilic mucoid stroma occupied the cores of small papillae, where stromal cells were rarely found (Figure 1a). In 12 cases, aggregation of the spherule-like mucoid stroma gave a cribriform appearance, as if each spherule-like mucoid stroma was a duct lumen filled with mucin. Some spherule-like mucoid stroma appeared to be intracellular mucin vacuoles. They were, however, neither duct lumina nor cell vacuoles. Immunohistochemistry for laminin γ2 chain showed that the rim of each spherule-like space was positive for laminin γ2 chain, indicating that they were actually subcellular spaces and pseudolumina (Figure 1b). This pattern was observed in areas showing either a papillary growth (9 cases) or a solid growth (3 cases). Mucin histochemistry revealed that the spherule-like mucoid stroma was negative for d-PAS but positive for alcian blue pH 2.5, and the positivity for alcian blue pH 2.5 was diminished after hyaluronidase digestion, indicating that the spherule-like mucoid stroma contained abundant glycosaminoglycan including hyaluronan. This contrasted to the mucin within the duct lumen, which was positive for both d-PAS and alcian blue pH 2.5, indicating that it was acidic glycoprotein (Figure 2).
Spherule-like mucoid stroma in clear cell carcinoma of the ovary. (a) The cores of small papillae are occupied by basophilic mucoid stroma, where fibroblastic cells are rarely found (H&E, left, × 200; right, × 400). (b) Either in areas showing a papillary architecture (upper) or areas showing a solid architecture (lower), the spherule-like mucoid stroma aggregates in a cribriform pattern, as if the mucoid spaces are true duct lumina. Some mucoid spaces appear to be intracellular mucin vacuoles. However, immunohistochemistry for laminin γ2 chain shows that the rims of the mucoid spaces are positive for laminin γ2 chain, indicating that they are not duct lumina or cell vacuoles, but subcellular spaces and therefore pseudolumina (left, H&E; right, laminin γ2 chain; × 400).
The spherule-like mucoid stroma in ovarian clear cell carcinoma is positive for alcian blue pH 2.5 (right upper), and the positivity is diminished after hyaluronidase digestion (right lower). It is negative for periodic acid-Schiff (PAS) before and after digestion by diastase (left lower). This contrasts to the mucin within the duct lumina (inset, arrowheads), which is positive for both PAS and alcian blue pH 2.5, regardless of digestion (left upper, H&E; left lower, PAS after diastase digestion; right upper, alcian blue pH 2.5; right lower, alcian blue pH 2.5 after hyaluronidase digestion; × 400).
The spherule-like mucoid stroma was frequently associated with hyalinized stroma in ovarian clear cell carcinomas. Among 29 clear cell carcinomas with spherule-like mucoid stroma, 26 (90%) were associated with hyalinized stroma. In total, 40 clear cell carcinomas had at least one type of stroma, and 26 clear cell carcinomas had both (Table 1). The spherule-like mucoid stroma and hyalinized stroma coexisted in some areas and were separated in other areas. In the former, they were often continuous with each other. There was a tendency for the mucoid stroma to be conspicuous if hyalinization scarcely occurred, and it was attenuated as hyalinization became more developed (Figure 3).
Coexistence of the spherule-like mucoid stroma and hyalinized stroma in ovarian clear cell carcinoma. (Upper) Mucoid change is more conspicuous than hyalinization. Some cores of papillae (asterisks) show a dual-colored target-like feature, where eosinophilic hyalinized material is distributed in the periphery, and basophilic mucoid material is distributed in the center. (Middle) Hyalinization dominates mucoid change. (Lower) A small amount of hyalinized stroma is found among an aggregation of spherule-like mucoid stroma (left, H&E; right, laminin γ2 chain; × 400).
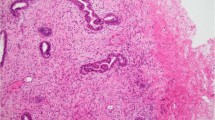
Among ovarian tumors other than clear cell carcinoma, 4 of 40 serous adenocarcinomas, 3 of 7 serous borderline tumors, and 1 of 15 mucinous borderline tumors focally showed mucoid stroma, mainly in the cores of papillae. It was, however, not of compact spherule-like appearance but of loose ill-defined appearance, and was usually associated with stromal cells (Figure 4). The cribriform distribution of mucoid stroma was not found in any of these tumors.
Cell Line
In vitro, HAC-2 formed spherule-like structures. Mucin histochemistry revealed that the structures contained glycosaminoglycan including hyaluronan in the center (Figure 5a). By latex agglutination immunoturbidimetry, a significant amount of hyaluronan (1400±84 ng/ml) was detected in the solution containing subcellular matrix, whereas <10 ng/ml of hyaluronan was detected in the control. By heterotransplantation into the abdominal cavity of nude mice, spherule-like mucoid stroma was found in the xenografts (Figure 5b).
(a) HAC-2, a clear cell carcinoma cell line, forms a spherule-like structure in vitro. The basophilic material in the center is positive for alcian blue, pH 2.5 (middle), and the positivity is reduced after digestion by hyaluronidase (right) (left, H&E; middle, alcian blue pH 2.5; right, alcian blue pH 2.5 after hyaluronidase digestion; × 400). (b) By heterotransplantation of HAC2 into the abdominal cavity of a nude mouse, spherule-like mucoid stroma is found in the xenograft (H&E, × 400).
Discussion
This study showed that spherule-like mucoid change, as well as hyalinization, is a characteristic stromal feature of ovarian clear cell carcinomas. The spherule-like mucoid stroma appeared as two distinct patterns. In the first pattern, basophilic mucoid stroma occupied the cores of small papillae, where stromal cells were rarely found. In the second pattern, the spherule-like mucoid stroma aggregated in a cribriform pattern, as if the mucoid spaces were true duct lumina. Some mucoid spaces appeared to be mucin vacuoles occupying the cytoplasm. They were, however, neither true duct lumina nor cell vacuoles. Immunohistochemistry for laminin γ2 chain confirmed that they were subcellular spaces and therefore pseudolumina. Such pseudolumina are analogous to pseudocysts in adenoid cystic carcinomas.5 Mucin histochemistry revealed that the spherule-like mucoid stroma contained abundant glycosaminoglycan including hyaluronan. In human tissue, one of the major sources of glycosaminoglycan is fibroblasts.6 In clear cell carcinomas, however, the glycosaminoglycan that accumulates in the spherule-like stroma is less likely to be produced by fibroblasts, because stromal cells are rarely found there. An earlier study showed that cell lines derived from adenoid cystic carcinoma produced glycosaminoglycan and formed pseudocysts in vitro.7 To examine whether clear cell carcinoma cells have the potential to synthesize glycosaminoglycan, we used HAC-2, a cell line derived from ovarian clear cell carcinoma. When HAC-2 was cultured in vitro, HAC-2 formed a spherule-like structure containing glycosaminoglycan (including hyaluronan) in the center. Production of hyaluronan by HAC-2 was also confirmed by latex agglutination immunoturbidimetry. Subsequent heterotransplantation of HAC-2 into nude mice showed that the xenografts had spherule-like mucoid stroma. All of these findings indicate that clear cell carcinoma cells are responsible for the production of glycosaminoglycan and the subsequent formation of spherule-like mucoid stroma. Further experimental studies, such as by inhibition of glycosaminoglycan production, are necessary to show the exact contribution of clear cell carcinoma cells in the formation of spherule-like mucoid stroma.
This study also showed that spherule-like mucoid stroma is strongly associated with hyalinized stroma. Among 40 clear cell carcinomas that had at least one type of stroma, 26 (65%) had both. The spherule-like mucoid stroma, as well as hyalinized stroma, was found far more frequently in areas showing a papillary architecture (25 cases) than in areas showing a solid architecture (4 cases). They coexisted in some areas and were separated in others. There was a tendency for the mucoid stroma to be conspicuous if hyalinization scarcely occurred, and it was attenuated as the hyalinization became more developed. In ovarian clear cell carcinomas, the hyalinized stroma is composed of basement membrane materials, including laminin-5 and type IV collagen, which are produced by tumor cells themselves.3, 8 It is most likely that the spherule-like mucoid stroma and hyalinized stroma symbolize a serial process of stromal remodeling, which is driven by stromal deposition of extracellular matrices produced by clear cell carcinoma cells. Again, this is fairly analogous to stromal remodeling in adenoid cystic carcinoma.9
From the viewpoint of practical diagnosis, the spherule-like mucoid stroma and hyalinized stroma are regarded as complementary findings of ovarian clear cell carcinomas. Although the hyalinized stroma is well recognized as a characteristic feature of clear cell carcinomas, it may only be focal or less developed. As described above, the spherule-like mucoid change is rather conspicuous in the stroma where the hyalinization scarcely develops, and this may support the diagnosis of clear cell carcinomas. Clear cell carcinomas may be misdiagnosed as serous borderline tumors or serous adenocarcinomas, especially in cases where clear cell carcinomas show a prominent papillary architecture. A recent report described several features that are most useful in distinguishing clear cell carcinomas from serous borderline tumors, including unilaterality or non-hierarchical branching.10 Focusing on stromal features is also useful, because they differ between clear cell carcinoma and serous tumor. In our series, 3 of 7 serous borderline tumors and 4 of 40 serous adenocarcinomas focally showed mucoid change in the stroma. It was, however, ill defined and associated with fibroblasts, suggesting that the fibroblasts are the probable source of the mucoid material. Cribriform aggregation of the mucoid stroma or association with basement membrane material was not found in any of these tumors. This study emphasizes that not only hyalinized stroma but also spherule-like mucoid stroma is to be taken into account in differential diagnosis of clear cell carcinoma.
It is now recognized that cancer cells cannot develop independently but can develop under interaction with surrounding stroma. During cancer development, the stroma is remodeled to support cancer cell proliferation, migration, invasion, or metastasis. In this context, it is interesting to know whether clear cell carcinoma cells take advantage of their remodeled stroma. In our earlier study in vitro, clear cell carcinoma cell lines on excess of laminin-5 increased their migratory activity by interaction between laminin-5 and their cell surface integrins or membrane-type matrix metalloproteinases, suggesting that hyalinized stroma containing abundant laminin-5 is responsible for increased migratory activity of clear cell carcinoma cells.11, 12 Glycosaminoglycan, a component of spherule-like mucoid stroma, consists of a heterogeneous group of polysaccharides, including hyaluronan, heparan sulfate, and chondroitin sulfate, and usually exists as a proteoglycan by binding with different kinds of proteins. As for hyaluronan, earlier studies showed that aberrant hyaluronan production and its peritumoral accumulation promotes the malignant behavior of cells, such as migration, invasion, or metastasis.13, 14, 15 In this study, three borderline clear cell tumors, none of which exhibited stromal invasion, also showed no stromal accumulation of either hyaluronan or laminin γ2 chain. Further study is needed to clarify the biological effects of hyaluronan and other glycosaminoglycans on clear cell carcinoma cells, and to resolve the issue of whether the coexistence of glycosaminoglycan and laminin-5 has a synergistic effect or anti-synergistic effect on clear cell carcinoma cells.
In conclusion, spherule-like mucoid change is a characteristic stromal feature of ovarian clear cell carcinomas, and is strongly associated with stromal hyalinization. Both spherule-like mucoid change and hyalinization are most likely to belong to a same spectrum of stromal remodeling, which is promoted by stromal deposition of extracellular matrices produced by clear cell carcinoma cells. The spherule-like mucoid stroma and hyalinized stroma are considered complementary diagnostic signatures of ovarian clear cell carcinomas.
References
Scully RE, Young RH, Clement PB . Atlas of Tumor Pathology. Tumors of the Ovary, Maldeveloped Gonads, Fallopian Tube, and Broad Ligament. Armed Forces Institute of Pathology: Washington, DC, 1998, pp 141–151.
Prat J . Pathology of the Ovary. Saunders: Philadelphia, 2004, pp 153–162.
Mikami Y, Hata S, Melamed J, et al. Basement membrane material in ovarian clear cell carcinoma: correlation with growth pattern and nuclear grade. Int J Gynecol Pathol 1999;18:52–57.
Benedet JL, Bender H, Jones III HW, et al. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO committee on Gynecologic Oncology. Int J Gynaecol Obstet 2000;70:209–262.
Ellis GL, Auclair PL . Atlas of Tumor Pathology. Tumors of the Salivary Glands. Armed Forces Institute of Pathology: Washington, DC, 1995, pp 203–216.
Laurent TC, Fraser JRE . Hyaluronan. FASEB J 1992;6:2397–2404.
Shirasuna K, Watatani K, Furusawa H, et al. Biological characterization of pseudocyst-forming cell lines from human adenoid cystic carcinomas of minor salivary gland origin. Cancer Res 1990;50:4139–4145.
Wong WSF, Wong YF, Ng YTA, et al. Establishment and characterization of a new human cell line derived from ovarian clear cell carcinoma. Gynecol Oncol 1990;38:37–45.
Cheng J, Saku T, Okabe H, et al. Basement membranes in adenoid cystic carcinoma. An immunohistochemical study. Cancer 1992;69:2631–2640.
Sangoi AR, Soslow RA, Teng NN, et al. Ovarian clear cell carcinoma with papillary features: a potential mimic of serous tumor of low malignant potential. Am J Surg Pathol 2008;32:269–274.
Kato N, Sasou S, Teshima S, et al. Overexpression of laminin-5 γ2 chain in clear cell carcinoma of the ovary. Virchows Arch 2006;450:273–278.
Kato N, Motoyama T . Relation between laminin-5 γ2 chain and cell surface metalloproteinase MT1-MMP in clear cell carcinoma of the ovary. Int J Gynecol Pathol 2009;28:49–54.
Itano N, Sawai T, Miyaishi O, et al. Relationship between hyaluronan production and metastatic potential of mouse mammary carcinoma cells. Cancer Res 1999;59:2499–2504.
Auvinen P, Tammi R, Parkkinen J, et al. Hyaluronan in peritumoral stroma and malignant cells associates with breast cancer spreading and predicts survival. Am J Pathol 2000;156:529–536.
Itano N, Atsumi F, Sawai T, et al. Abnormal accumulation of hyaluronan matrix diminishes contact inhibition of cell growth and promotes cell migration. Proc Natl Acad Sci USA 2002;99:3609–3614.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kato, N., Takeda, J., Fukase, M. et al. Alternate mucoid and hyalinized stroma in clear cell carcinoma of the ovary: manifestation of serial stromal remodeling. Mod Pathol 23, 881–888 (2010). https://doi.org/10.1038/modpathol.2010.75
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2010.75