Abstract
Hyaluronan (HA) accumulation has been associated with poor survival in various cancers, but the mechanisms for this phenomenon are still unclear. The aim of this study was to investigate the prognostic significance of stromal HA accumulation and its association with host immune response in pancreatic ductal adenocarcinoma (PDAC). The study material consisted of 101 radically treated patients for PDAC from a single geographical area. HA staining was evaluated using a HA-specific probe, and the patterns of CD3, CD8, CD73 and PD-L1 expression were evaluated using immunohistochemistry. HA staining intensity of tumour stromal areas was assessed digitally using QuPath. CD3- and CD8-based immune cell score (ICS) was determined. High-level stromal HA expression was significantly associated with poor disease-specific survival (p = 0.037) and overall survival (p = 0.013) In multivariate analysis, high-level stromal HA expression was an independent negative prognostic factor together with histopathological grade, TNM stage, CD73 positivity in tumour cells and low ICS. Moreover, high-level stromal HA expression was associated with low ICS (p = 0.017). In conclusion, stromal HA accumulation is associated with poor survival and low immune response in PDAC.
Similar content being viewed by others
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive solid malignancies with 5-year survival rates of 2–9%1,2. This is partly related to advanced disease stage at the time of diagnosis ruling out curative surgery. Tumour cells are in constant interaction with non-neoplastic cells, and the tumour microenvironment influences cancer progression. PDAC has been shown to develop mechanisms that suppress the host immune response against the tumour3. The first signs of this immune suppression are seen already in the premalignant lesions4. PDAC is characterized by an abundant desmoplastic stroma, which has been suggested to facilitate the escape from the immune surveillance5,6.
Hyaluronan (HA) is one of the main components of the extracellular matrix. In normal physiological conditions, it is strongly expressed during wound healing and at sites of inflammation, including cancer7. It also influences immune responses8. A complex regulation system controls HA metabolism, mainly dependent on HA-producing synthases and degrading hyaluronidases. The activation of the cell surface HA receptors such as CD449 and RHAMM10 modulate cell proliferation, aggregation, migration and angiogenesis11 and may also be involved in the HA-induced epithelial–mesenchymal transition12 and stem cell functions13. HA has been shown to be overexpressed in most human malignancies14,15,16,17,18,19,20,21,22,23. Several studies have indicated that hyaluronan accumulation in the tumour cells and/or peritumoral stroma is related to tumour progression and poor survival in many cancer types23,24,25,26,27,28,29,30,31. However, the mechanisms underlying the association between accumulation of HA, host immune response and poor survival remain unclear, especially in PDAC.
The association between a strong immune response and better survival is well established in various cancers32,33,34,35,36. We have previously introduced a T-lymphocyte-based immune cell score (ICS) as a strong favourable prognostic factor in PDAC37. Extensive alterations occur in the complex PDAC microenvironment during the tumorigenesis. Multiple mechanisms, such as overexpression of the immunosuppressive molecules CD73 and PD-L1, may lead to immune suppression38,39,40,41. There is some evidence showing that HA plays a role in immune response regulation42,43. According to our hypothesis this might be one of the key factors explaining the association between HA accumulation and low survival among cancer patients25.
The aim of the present study was to examine the prognostic role of stromal HA accumulation and its relation to immune cell infiltration and the immune-suppressing molecules CD73 and PD-L1 in PDAC.
Methods
Patients
From 2000 to 2016, a total of 129 patients with PDAC were operated on with curative intent in the Central Hospital of Central Finland, Jyväskylä, Finland. Patients with locally inoperable tumour, peritoneal carcinosis or distant metastases were excluded, resulting in the 101 patients with stage IA-IIB disease. Detailed information on patient and tumour characteristics, surgical treatment and complications, oncological treatment and follow-up were collected prospectively, updated and confirmed by a review of patient records review. None of the included patients received neoadjuvant chemotherapy before surgery.
Histopathological examination
All histopathological tumour specimens were reviewed by an experienced gastrointestinal histopathologist (JB). Tumour staging was done according to the 7th edition of the UICC/AJCC TNM categories44. The grading was performed according to the WHO classification of tumours 201045.
Tumour sampling, HA assay, and immunohistochemistry
Tissue microarray (TMA) blocks were constructed as described previously, from formalin-fixed paraffin-embedded primary PDAC patient tumour samples. Two tissue cores 0.6 mm in diameter were taken both from the core of the tumour and the invasive margin from representative tumour blocks. Sections of 2 µm thickness were used for immunohistochemical (IHC) analyses37.
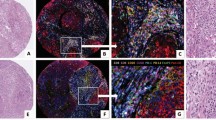
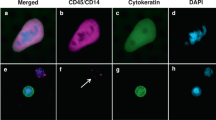
Hyaluronan was stained as described previously26. Briefly, a complex containing the G1 domain of cartilage aggrecan and link protein was labeled with biotin (bHABC), diluted to 3 µg/ml of 1% bovine serum albumin in phosphate buffer, and incubated overnight at 4 °C on sections pretreated with H2O2 and 1% bovine serum albumin to block endogenous peroxidases and unspecific binding, respectively. After one hour incubation in avidin–biotin–peroxidase (Vector Laboratories, Irvine, CA; 1:200 dilution) the sections were washed with PBS, and incubated in 0.05% 3,3′-diaminobenzidine (Sigma Chemical Co., St. Louis, MO) and 0.03% H2O2 in the phosphate buffer, followed by nuclear counterstaining with Mayers hematoxylin (Fig. 1). Staining for CD73 was conducted as described previously, with rabbit monoclonal anti-CD73 antibody (D7F9A, Cell Signalling) and ultraView Universal DAB detection kit (Roche) for Ventana39 (Fig. 2). Staining for CD3 and CD8 was conducted with anti-CD3 (LN 10, 1:200; Novocastra) and anti-CD8 (SP16, 1:400; Thermo Scientific) antibodies, using a Lab Vision Autostainer 480 (Immunovision Technologies Inc.) (Fig. 3). Staining for PD-L1 was conducted as described previously, with anti-PD-L1 (E1L3N, 1:100; Cell Signalling Technology) antibody, using a BOND-III stainer (Leica Biosystems). PD-L1 staining was carried out using whole tissue sections 37 (Fig. 4).
Signal visualization for all IHC was done by diaminobenzidine and sections were counterstained with haematoxylin.
In general, HA staining was clearly seen in all specimen both in stroma and in tumour epithelium. Stained TMA sections were scanned using an Aperio digital slide scanner (Leica Biosystems), followed by analysis using QuPath v 0.1.2 as described below.
ICS was determined using the TMA technique as described earlier37. Briefly, ICS describes the immune response represented by the density of CD3 and CD8 positive immune cells in the tumour centre and at the invasive margin. PD-L1 expression was evaluated by estimating the proportion of PD-L1 positivity on the tumour cell surface as described earlier39. In addition, we analyzed also the proportion of PD-L1 expression in stromal cells using the 5% staining proportion as a cutoff.
Quantitative evaluation of HA staining
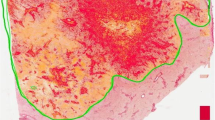
HA was evaluated using QuPath v 0.1.246. First, the stain vectors and background values were estimated using the Estimate stain vectors command to facilitate stain separation with the color deconvolution method. Simple tissue detection command was used to delineate the tissue area from the white background. This area was manually edited with the brush tool to exclude tumour epithelial regions. SLIC superpixel segmentation was used to divide the area into superpixels (neighboring groups of pixels sharing similar characteristics). DAB intensity was calculated for each superpixel, and the data were exported at individual superpixel level. R statistical programming language version 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria) was used to summarize the mean intensity for each case. The distribution of DAB intensities was similar in different TMAs suggesting that the assay had performed uniformly (Fig. S.1). The cores from tumour centre and invasive margin had a strong positive correlation (Spearman’s rho = 0.69), and average HA intensity of all available tumour regions was therefore used in the main analyses.
Samples were divided into two groups based on the mean intensity value: high and low stromal HA expression. To determine cut-off values for HA expression with optimal sensitivity and specificity, we used receiver operating characteristic (ROC) curve drawn in relation to disease-specific 3-year mortality.
Statistical analyses
The chi-square test was used when analysing the associations between HA and clinical and histopathological variables, CD73 positivity and PD-L1 positivity in tumour cells. The estimates for hazard ratios for overall survival (OS) and disease specific survival (DSS) were calculated using univariate and multivariate Cox proportional hazards regression model. Only variables with p < 0.05 in univariate analysis were entered into the multivariate analysis despite the a-priori determined confounder, tumour stage (p = 0.117). All statistical tests were two-sided. A p value less than 0.05 was considered significant. The statistical analyses were performed and Fig. 5 created with IBM SPSS statistics 24 for Windows (IBM Corporation, Armonk, NY, USA, https://www.ibm.com/analytics/spss-statistics-software).
Compliance with ethical standards
The use of patient samples and the data inquiry were approved by the Oulu University Hospital Ethics Committee. The need to obtain written or oral consent from patients to use their samples in research was waived by the Finnish National Authority for Medicolegal Affairs (VALVIRA, Dnro 10,832/06.01.03.01/2014). This study was designed and performed according to the reporting recommendations for tumour marker prognostic studies (REMARK) and the Declaration of Helsinki47,48.
Results
Patient demographics
A total of 101 of PDAC patients were included in this study. The distribution of key clinicopathological variables among these patients is shown in Table 1.
Associations between stromal HA expression and other histopathological variables
Stromal HA accumulation appeared to associate with low ICS (p = 0.017). The associations between stromal HA expression and other clinical and histopathological variables, cell-specific CD73 positivity and PD-L1 positivity in tumour cells were also assessed and are shown in Table 2. Stromal HA accumulation was not associated with other clinicopathological parameters, including CD73 positivity in tumour cells and PD-L1 positivity in tumour cells and stromal cells.
Stromal HA accumulation and survival
Regarding the whole study group, the median follow-up time was 44 (IQR 15.0 to 57.0) months for those alive at the end of follow-up. The estimated median OS for all patients was 25 months [95% CI: (17.7–32.3)]. Stromal HA accumulation was significantly associated with poor DSS (p = 0.037) and OS (p = 0.013) (Fig. 5).
In the multivariate analysis, stromal HA accumulation was an independent negative prognostic factor together with histopathological grade, TNM stage, CD73 positivity in tumour cells and low ICS (Table 3).
Discussion
In the present study, using a larger, consecutive patient series from a single geographical area of Northern Finland without apparent selection bias, we showed the role of stromal HA accumulation as an independent prognostic factor for poor survival in pancreatic cancer. We also found an association between the HA accumulation and low immune response as judged by the tumour-infiltrating T-cell densities.
While the number of patients in the present work was higher than in previous studies on HA in PDAC6,24, an even larger material would probably have allowed a connection between T-cell score and hyaluronan stronger than that now established. The small tissue cores turned out to be quite acceptable for the analysis since in preliminary tests the HA intensities were strongly correlated between different cores of the same tumour even between the tumour centre and invasive margin. The computer-assisted evaluation of HA staining adopted for the present work was felt easier than manual scoring of sometimes minor differences in intensity. It can also be recommended for future studies due to its independence of personal variation between evaluators.
Stromal HA accumulation in malignancies originating from non-stratified epithelium is associated with a poor survival in a number of solid tumours7. Indeed, given the large desmoplastic stroma in PDAC, a major role of HA was expected in the progression of this cancer. The idea was further supported for example by a fact that a drug specifically reducing HA synthesis inhibits human PDAC cell growth in vitro and in mice in vivo49. However, clinical trials combining enzymatic removal of HA and cytostatic drugs have been disappointing50,51 suggesting that it is not just the content of HA that enhances malignant growth. Rather, activated synthesis and concurrent degradation of HA probably provide an environment supporting cancer spreading7. This becomes understandable by considering the two opposite influences of HA on cell migration. By its swelling pressure HA gel creates free space for cells to move in, while at the same time blocks attachment to adjacent cells and matrix proteins.
Indeed, the cell surface hyaluronidase TMEM2 is an independent negative prognostic factor in PDAC52, demonstrating the importance of HA degradation in PDAC progression. TMEM2 associates to integrins and clears HA to facilitate cancer cell adhesion and migration53. Besides facilitating migration in HA-rich matrix the fragments created by hyaluronidase act as a signal that amplify inflammation.
Increasing numbers of studies have shown the impact of HA on the host immune response: It is suggested to protect tumour cells against immune attack by forming peri-cellular coats54. Moreover, HA accumulation seems to facilitate tumour-associated macrophage infiltration and their differentiation into the pro-tumoral M2 phenotype25,42 with an immunosuppressive effect preventing antitumour immunity by T-cells. Interestingly, in the present study we demonstrate an inverse correlation between T-cell-based ICS and stromal HA accumulation in PDAC. This supports the idea that an HA-rich extracellular matrix not only acts as a shield between T-cells and tumour cells, but also prevents T-cell infiltration in the whole tumour microenvironment. We have previously published a paper showing that CD73 positivity in PDAC cells is a prognostic factor in PDAC independently of ICS and hypothesized that CD73 suppresses immune response by impacting on the activity of the tumour infiltrating lymphocytes rather than their number39. This might also explain why stromal HA expression associates with ICS but not with CD73 or PD-L1.
Recently, different phenotypes of ECM-producing cancer-associated fibroblasts (CAFs) have been described, including inflammatory CAFs and myofibroblastic CAFs55. Inflammatory CAFs are supposed to be tumour-promoting via immune suppression56. In future, it would be reasonable to find out if the HA accumulation associates with the polarization of CAFs, since this would further give some insight into the potential mechanism behind the association between HA and immune status. One possible link between CAF polarisation and HA synthesis is the STAT3-signaling pathway, since the inhibition of STAT3—pathway has been shown to downregulate HA—synthesis57 and, on the other hand, to promote differentiation of CAFs into myCAFs56.
As far as we know, the association of HA on T-cell immune response has not been studied earlier in PDAC but the present finding clearly warrants further expansion of the studies to obtain a more detailed view of the interactions between HA and lymphocytes in this disease with such a bleak prognosis. In future studies, information of physical properties (for example molecular mass) of HA molecules is also needed, since there are data indicating that molecular weight affects the biological functions of HA molecules58.
In conclusion, our study indicates that stromal HA accumulation may be associated with low T cell densities in the PDAC microenvironment, but still represents an adverse prognostic parameter independent of T cell densities, tumour stage, tumour grade, and CD73 expression. The results warrant further definition of the interactions between T-cell immunity and hyaluronan in the tumour microenvironment.
Abbreviations
- HA:
-
Hyaluronan
- ICS:
-
Immune cell score
- PDAC:
-
Pancreatic ductal adenocarcinoma
- DSS:
-
Disease-specific survival
- OS:
-
Overall survival
- TMA:
-
Tissue microarray
References
Luo, J., Xiao, L., Wu, C., Zheng, Y. & Zhao, N. The incidence and survival rate of population-based pancreatic cancer patients: Shanghai Cancer Registry 2004–2009. PLoS ONE 8(10), e76052 (2013).
Ferlay, J. et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136(5), E359–E386 (2015).
Ene-Obong, A. et al. Activated pancreatic stellate cells sequester CD8+ T cells to reduce their infiltration of the juxtatumoral compartment of pancreatic ductal adenocarcinoma. Gastroenterology 145(5), 1121–1132 (2013).
Hiraoka, N., Onozato, K., Kosuge, T. & Hirohashi, S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin. Cancer Res. 12(18), 5423–5434 (2006).
Tammi, R. H. et al. Hyaluronan in human tumors: Pathobiological and prognostic messages from cell-associated and stromal hyaluronan. Semin Cancer Biol. 18(4), 288–295 (2008).
Whatcott, C. J. et al. Desmoplasia in primary tumors and metastatic lesions of pancreatic cancer. Clin. Cancer Res. 21(15), 3561–3568 (2015).
Tammi, M. I. et al. Activated hyaluronan metabolism in the tumor matrix—Causes and consequences. Matrix Biol. 78–79, 147–164 (2019).
Kuipers, H. F. et al. Hyaluronan synthesis is necessary for autoreactive T-cell trafficking, activation, and Th1 polarization. Proc. Natl. Acad. Sci. U. S. A. 113(5), 1339–1344 (2016).
Aruffo, A., Stamenkovic, I., Melnick, M., Underhill, C. B. & Seed, B. CD44 is the principal cell surface receptor for hyaluronate. Cell 61(7), 1303–1313 (1990).
Turley, E. A. Hyaluronan and cell locomotion. Cancer Metastasis Rev. 11(1), 21–30 (1992).
Nikitovic, D., Tzardi, M., Berdiaki, A., Tsatsakis, A. & Tzanakakis, G. N. Cancer microenvironment and inflammation: Role of hyaluronan. Front. Immunol. 6, 169 (2015).
Porsch, H. et al. Efficient TGFβ-induced epithelial-mesenchymal transition depends on hyaluronan synthase HAS2. Oncogene 32(37), 4355–4365 (2013).
Chanmee, T. et al. Hyaluronan production regulates metabolic and cancer stem-like properties of breast cancer cells via hexosamine biosynthetic pathway-coupled HIF-1 signaling. J. Biol. Chem. 291(46), 24105–24120 (2016).
Llaneza, A. et al. Hyaluronic acid as prognostic marker in resectable colorectal cancer. Br. J. Surg. 87(12), 1690–1696 (2000).
Theocharis, A. D., Vynios, D. H., Papageorgakopoulou, N., Skandalis, S. S. & Theocharis, D. A. Altered content composition and structure of glycosaminoglycans and proteoglycans in gastric carcinoma. Int. J. Biochem. Cell Biol. 35(3), 376–390 (2003).
García, I. et al. Relationship of tumoral hyaluronic acid and cathepsin D contents with histological type of gastric carcinoma. Int. J. Biol. Markers 15(3), 215–218 (2000).
Hopwood, J. J. & Dorfman, A. Glycosaminoglycan synthesis by Wilms’ tumor. Pediatr. Res. 12(1), 52–56 (1978).
Roboz, J., Greaves, J., Silides, D., Chahinian, A. P. & Holland, J. F. Hyaluronic acid content of effusions as a diagnostic aid for malignant mesothelioma. Cancer Res. 45(4), 1850–1854 (1985).
Fukunaga, A. et al. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas 28(1), e26-31 (2004).
Horai, T., Nakamura, N., Tateishi, R. & Hattori, S. Glycosaminoglycans in human lung cancer. Cancer 48(9), 2016–2021 (1981).
Takeuchi, J. et al. A high level of glycosaminoglycan-synthesis of squamous cell carcinoma of the parotid gland. Cancer 47(8), 2030–2035 (1981).
Kojima, J., Nakamura, N., Kanatani, M. & Omori, K. The glycosaminoglycans in human hepatic cancer. Cancer Res. 35(3), 542–547 (1975).
Lipponen, P. et al. High stromal hyaluronan level is associated with poor differentiation and metastasis in prostate cancer. Eur. J. Cancer 37(7), 849–856 (2001).
Cheng, X. B., Sato, N., Kohi, S. & Yamaguchi, K. Prognostic impact of hyaluronan and its regulators in pancreatic ductal adenocarcinoma. PLoS ONE 8(11), e80765 (2013).
Tiainen, S. et al. High numbers of macrophages, especially M2-like (CD163-positive), correlate with hyaluronan accumulation and poor outcome in breast cancer. Histopathology 66(6), 873–883 (2015).
Auvinen, P. et al. Hyaluronan in peritumoral stroma and malignant cells associates with breast cancer spreading and predicts survival. Am. J. Pathol. 156(2), 529–536 (2000).
Ropponen, K. et al. Tumor cell-associated hyaluronan as an unfavorable prognostic factor in colorectal cancer. Cancer Res. 58(2), 342–347 (1998).
Setälä, L. P. et al. Hyaluronan expression in gastric cancer cells is associated with local and nodal spread and reduced survival rate. Br. J. Cancer 79(7–8), 1133–1138 (1999).
Köbel, M. et al. Epithelial hyaluronic acid and CD44v6 are mutually involved in invasion of colorectal adenocarcinomas and linked to patient prognosis. Virchows Arch. 445(5), 456–464 (2004).
Anttila, M. A. et al. High levels of stromal hyaluronan predict poor disease outcome in epithelial ovarian cancer. Cancer Res. 60(1), 150–155 (2000).
Böhm, J. et al. Hyaluronan expression in differentiated thyroid carcinoma. J. Pathol. 196(2), 180–185 (2002).
Anitei, M. G. et al. Prognostic and predictive values of the immunoscore in patients with rectal cancer. Clin. Cancer Res. 20(7), 1891–1899 (2014).
Hatogai, K. et al. Comprehensive immunohistochemical analysis of tumor microenvironment immune status in esophageal squamous cell carcinoma. Oncotarget 7(30), 47252–47264 (2016).
Jiang, W. et al. Tumor-infiltrating immune cells and prognosis in gastric cancer: A systematic review and meta-analysis. Oncotarget 8(37), 62312–62329 (2017).
Wang, M. ImmunoScore predicts gastric cancer postsurgical outcome. Lancet Oncol. 18(2), e68 (2017).
Fridman, W. H., Pages, F., Sautes-Fridman, C. & Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. 12(4), 298–306 (2012).
Tahkola, K. et al. High immune cell score predicts improved survival in pancreatic cancer. Virchows Arch. 472(4), 653–665 (2018).
Zhuan-Sun, Y. et al. Prognostic value of PD-L1 overexpression for pancreatic cancer: Evidence from a meta-analysis. Onco Targets Ther. 10, 5005–5012 (2017).
Tahkola, K. et al. Prognostic impact of CD73 expression and its relationship to PD-L1 in patients with radically treated pancreatic cancer. Virchows Arch. 478(2), 209–217 (2020).
Clark, A. G. & Vignjevic, D. M. Modes of cancer cell invasion and the role of the microenvironment. Curr. Opin. Cell Biol. 36, 13–22 (2015).
Kim, R., Emi, M. & Tanabe, K. Cancer immunoediting from immune surveillance to immune escape. Immunology 121(1), 1–14 (2007).
Kuang, D.-M. et al. Tumor-derived hyaluronan induces formation of immunosuppressive macrophages through transient early activation of monocytes. Blood 110(2), 587–595 (2007).
Iijima, J., Konno, K. & Itano, N. Inflammatory alterations of the extracellular matrix in the tumor microenvironment. Cancers (Basel) 3(3), 3189–3205 (2011).
Edge, S. B. et al. (eds) AJCC Cancer Staging Manual (Springer, 2010).
Bosman, F. T. et al. (eds) WHO Classification of Tumours of the Digestive System (WHO, 2010).
Bankhead, P. et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 7(1), 16878 (2017).
World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 310(20), 2191–2194 (2013).
McShane, L. M. et al. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br. J. Cancer 93(4), 387–391 (2005).
Hajime, M. et al. Inhibitory effect of 4-methylesculetin on hyaluronan synthesis slows the development of human pancreatic cancer in vitro and in nude mice. Int. J. Cancer 120(12), 2704–2709 (2007).
Ramanathan, R. K. et al. Phase IB/II randomized study of FOLFIRINOX plus pegylated recombinant human hyaluronidase versus FOLFIRINOX alone in patients with metastatic pancreatic adenocarcinoma: SWOG S1313. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 37(13), 1062–1069 (2019).
Van Cutsem, E. et al. Randomized phase III trial of pegvorhyaluronidase alfa with nab-paclitaxel plus gemcitabine for patients with hyaluronan-high metastatic pancreatic adenocarcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 38(27), 3185–3194 (2020).
Kudo, Y. et al. Overexpression of transmembrane protein 2 (TMEM2), a novel hyaluronidase, predicts poor prognosis in pancreatic ductal adenocarcinoma. Pancreatol. Off. J. Int. Assoc. Pancreatol. 20(7), 1479–1485 (2020).
Irie, F. et al. The cell surface hyaluronidase TMEM2 regulates cell adhesion and migration via degradation of hyaluronan at focal adhesion sites. J. Biol. Chem. 296, 100481 (2021).
Gately, C. L. et al. In vitro studies on the cell-mediated immune response to human brain tumors. II. Leukocyte-induced coats of glycosaminoglycan increase the resistance of glioma cells to cellular immune attack. J. Immunol. 133(6), 3387–3395 (1984).
Öhlund, D. et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med. 214(3), 579–596 (2017).
Biffi, G. et al. IL1-induced JAK/STAT signaling is antagonized by TGFβ to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov. 9(2), 282–301 (2019).
King, A. et al. Interleukin-10 regulates the fetal hyaluronan-rich extracellular matrix via a STAT3-dependent mechanism. J. Surg. Res. 184(1), 671–677 (2013).
Jiang, D., Liang, J. & Noble, P. W. Hyaluronan as an immune regulator in human diseases. Physiol. Rev. 91(1), 221–264 (2011).
Acknowledgements
We thank Eija Rahunen for her excellent assistance in HA stainings.
Funding
This study received funding from the Cancer Foundation Finland, the Finnish Medical Foundation, the Jane and Aatos Erkko Foundation, the Emil Aaltonen Foundation and the State Research Funding.
Author information
Authors and Affiliations
Contributions
All authors met the criteria listed in the ICMJE recommendations for the qualification of authorship. K.T. and J.B. had full access to all the data and take responsibility for the integrity of the data and the accuracy of the analysis. Concept and design: K.T., J.M. and J.B. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: K.T., J.V. and J.B. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: K.T. and J.B. Administrative, technical, or material support: All authors. Supervision: J.M., J.L., I.K. and J.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tahkola, K., Ahtiainen, M., Mecklin, JP. et al. Stromal hyaluronan accumulation is associated with low immune response and poor prognosis in pancreatic cancer. Sci Rep 11, 12216 (2021). https://doi.org/10.1038/s41598-021-91796-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-91796-x
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.