Abstract
The 2004 WHO classification of thymic tumors recognizes five major subtypes of thymomas and thymic carcinoma. Subtypes A and AB thymomas are purported to be benign neoplasms, although prior studies have suggested a potential for malignant behavior. The purpose of this study was to assess the clinical behavior of A and AB thymomas identified from a large institutional pathologic database. A retrospective slide review of 500 thymic epithelial tumors identified 71 (∼14%) cases of types A and AB thymomas. Clinical history and follow-up information were obtained through retrospective chart review. There were 38 and 33 cases of types A and AB thymomas, respectively. Complete follow-up data were available in 37 (52%) cases. Eighteen (49%) patients (type A, n=9 and type AB, n=9) had evidence of recurrent/metastatic disease at an average of 62 months (range from 6 to 244 months) after initial diagnosis. Survival curves for patients with types A and AB thymomas, with and without recurrences, show a statistically significant difference (P=0.001 and 0.005, respectively). Analysis of this large cohort confirms the potential for subtypes A and AB thymomas to show malignant behavior. Long-term clinical monitoring, therefore, appears to be justified in these cases. This study also shows the poor correlation between the WHO classification and tumor behavior
Similar content being viewed by others
Main
Thymomas are rare tumors that arise from the epithelium of the thymic gland.1 The rarity, histologic diversity, variable clinical behavior, and outcome have significantly limited our understanding of these tumors. Considerable and significant controversy continues to exist concerning the classification and clinical behavior of these tumors.
Characteristically admixed with a variable amount of lymphocytes (including no lymphocytes), thymomas are capable of rather diverse histopathology. This has resulted in a confusing array of classification schemas. Early work by Lattes2 divided these lesions based on the predominant cell type into lymphocytic, epithelial, mixed, and spindle types.
In 1978, Levine and Rosai1 postulated that encapsulated thymomas are essentially benign. This was based on the observation that most thymomas tend to be circumscribed and curable when amenable to complete surgical extirpation. Moreover, this held true irrespective of the histological features.1 Thymomas were deemed malignant when invasive growth was present or sufficient cellular anaplasia was present to reflect the presence of carcinoma.
On the basis of the purported morphologic similarity with thymic cortical or medullary epithelium, the Muller-Hermelink classification recognized five subtypes: medullary, mixed, predominantly cortical, and cortical, as well as well-differentiated thymic carcinoma.3, 4 In 1999, the WHO introduced an unifying schema that consisted of six types: A, AB, B1, B2, B3, and C.5 Type A tumors were composed of spindle or oval epithelial cells, whereas type B tumors consisted of stellate or polygonal epithelial cells. Type B tumors, which by definition lack the atypia of carcinoma, were further divided into three subtypes (B1, B2, and B3) based on the relative proportion of neoplastic epithelial cells and lymphocytes. Type AB tumors consisted of type A with areas that resembled type B. Type C tumors were defined by the presence of significant cellular anaplasia (ie frank carcinoma). In the revised 2004 version of WHO, the term ‘thymic carcinoma’ replaced the designation type C.6 In addition, a micronodular variant, which could occur in ‘pure’ form or in combination with other subtypes, was recognized.
The 2004 WHO schema considers types A and AB thymomas as benign lesions.6 This is disputed by others, who cite numerous studies documenting recurrence and death in patients with these subtypes.7, 8, 9 To re-examine this issue and better characterize the biologic behavior of these subtypes, we analyzed the recurrence patterns of WHO types A and AB thymomas evaluated at the Indiana University Melvin and Bren Simon Cancer Center (IUSCC), a tertiary cancer center.
Materials and methods
IUSCC evaluates a large number of thymoma patients, as clinical referrals, to two of the authors (PL and KK). As a result, we have slides and/or blocks from over 500 cases of thymic neoplasms treated between 1989 and 2009. Following Institutional Review Board approval, a retrospective slide review by two of the authors (SB and JH) identified 71 cases of WHO types A and AB thymomas.
The clinical information and follow-up data were acquired by chart review; as majority of the patients received initial care at an outside institution, complete clinical history and operative notes were not always available. Recorded variables included age, gender, type of treatment, complete or incomplete resection, tumor size, histologic subtype, Masaoka tumor stage,10 clinical features, and presence of autoimmune disease such as myasthenia gravis. Age was categorized as ≤60 and >60 and tumor size as ≤8 and >8 cm.11
The association between recurrence status and histology with categorical variables and continuous variables was analyzed using the χ2 and Mann–Whitney tests, respectively. Survival data of patients with recurrence/metastasis were compared with disease-free patients of types A and AB thymomas using the log-rank test. Survival curves were plotted using Kaplan–Meier method. Statistical analyses were performed using SPSS v17.0 with 0.05 as the significance level.
Results
Patient Characteristics
The overall male to female ratio was 1:1.4 with patient age ranging from 28 to 85 years (median: 62 years) (Table 1 ). The median age of patients with type A tumors was significantly higher than those with type AB tumors, 65 vs 55 years, respectively (P=0.012).
Clinical Findings
Clinical presentation history was available for 52 patients (27 of type A and 25 of type AB) and summarized in Table 2 and Supplementary Tables 1a and 2a.
Seventeen type A cases presented predominantly with chest-related symptoms such as dyspnea (eight), chest pain (six), or upper respiratory infection (URI) (three). One of the six patients with chest pain also had pneumonia. Five patients had myasthenia gravis and presented with ptosis, diplopia, or difficulty swallowing. Three patients also had a second tumor (one lung and two ovarian carcinomas). Three patients had other chronic diseases including hypertension (three), diabetes mellitus (one), and coronary artery disease (one) (Table 2; Supplementary Table 1a).
Eighteen patients with type AB tumors presented with chest-related symptoms including dyspnea (five), chest pain (seven), URI (four), and cough/pneumonia (two). One patient presented with fatigue as the only symptom. Three patients presented with symptoms of myasthenia gravis. Two patients had autoimmune disease: red cell aplasia (one) and systemic lupus erythematosus (one). Two patients also had a history of a second cancer: seminoma and breast carcinoma. Nine patients had other chronic conditions such as hypertension (five), renal insufficiency (two), diabetes mellitus (one), and Parkinson disease (one) (Table 2; Supplementary Table 2a).
In seven patients (four type A and three mixed AB), the diagnosis of thymoma was incidental to routine chest X-rays or computed tomography scans.
Macroscopic Features
All tumors were nodular, gray-white, and fleshy on cut section. Tumor size was available in 47 cases.
Regarding the 23 type A tumors, the median size was 6 cm (range: 2.5–19 cm). Of the 17 cases with a detailed gross description, 14 were encapsulated with two of these having foci of hemorrhagic necrosis and two others showing cystic change. Three other tumors were described as either multicystic or soft tissue-like. One of these invaded the lung and was adherent to the pericardium, whereas another invaded the wall of the innominate vein and adhered to the lung.
Among the 24 type AB tumors, the median size was 5.7 cm (range: 2.2–26 cm). Fifteen of the 18 tumors with detailed gross description were encapsulated. Two of these also exhibited areas of hemorrhage and necrosis. Three tumors invaded lung with one showing additional involvement of pericardium and large veins.
Histopathologic Findings
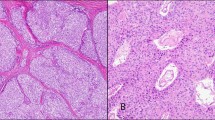
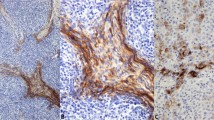
The 38 type A thymomas consisted of spindle- or oval-shaped epithelial cells lacking nuclear atypia. These tumors were devoid of a lymphocytic infiltrate (Figure 1a). In four cases, foci of micronodular variant thymoma were identified. In two tumors, areas of thymic carcinoma were additionally recognized. The 33 type AB thymomas consisted of type A thymoma features with a variably dense lymphocytic component (Figure 1b). Immunohistochemical stains for a number of lymphocytic markers (including CD5), cytokeratins, and CD117 had been performed at referring institutions (as per reports) to exclude the possibility of thymic carcinomas.
In six cases, biopsy confirmation of metastatic disease was performed; of these, slides from primary and metastatic lesions were available for review in two cases. These showed identical histological features; specifically, ‘malignant’ transformation was not noted at the metastatic site.
Stage at Diagnosis
Thirteen cases of type A were stage I, eleven stage II, seven stage III, one stage IV, and staging was unknown for six cases at the time of diagnosis. Fifteen cases of type AB were stage I, ten stage II, four stage III, two stage IV, and unknown for two cases at the time of diagnosis.
Treatment Data
As most of the patients were primarily treated at outside institutions, data with regards to treatment details were extracted from referral notes.
Treatment data were available for 36 of the 38 type A patients. ‘Complete’ surgical excision was performed in all patients with additional adjuvant chemotherapy (three patients), radiotherapy (three patients), or both (two patients). Three patients had received neoadjuvant chemotherapy with post-resection radiotherapy.
Thirty of the 32 patients with type AB tumors with treatment details available were treated with surgery, either alone (22) or in combination with neoadjuvant (three), adjuvant (two) chemotherapy, or radiotherapy (three). One of the remaining two patients had partial resection with chemotherapy, whereas the other received chemotherapy only. The presence of intraoperative adhesions was noted in only four patients (two patients in each group) treated with surgery.
Survival Analysis
Follow-up information was available for 37 patients (20 of type A and 17 of type AB) and ranged from 1 to 246 months (median: 30 months). Ten of the 20 patients with type A tumors are alive with no evidence of disease. One patient had metastatic pleural disease at the time of initial diagnosis. Nine patients developed recurrent and/or metastatic disease after a mean duration of 61.8 months (range: 6 months to 20 years) (Table 2). The sites involved in patients with single metastatic events were pleura (one), brain (one), peritoneum (one), and lung (three) with concomitant liver metastases (one). Two patients had multiple metastases involving lung, pleura, liver, and bone. Biopsy confirmation of metastatic thymoma occurred in three patients.
Of the 17 patients with type AB thymoma, eight were free of disease at last follow-up and nine developed recurrent or metastatic cases. Four patients had solitary metastases to pleura (two) or lung (two). Two patients had pleural metastases with additional involvement of lung or subcutaneous tissue of the back. Metastasis to the liver and gallbladder occurred in one patient. Two patients had multiple metastatic events: one with lung, pleura, and liver involvement and the other with lung, pleura, diaphragm, and renal involvement. The mean time to recurrence or metastasis in these patients was 62.25 months (range: 12–138 months) (Table 2). Two patients had thoracic metastases (pleura and lung; one each) at the time of initial diagnosis. Metastases were confirmed by biopsy in three patients.
Three patients with metastatic disease at time of diagnosis were excluded from analyses of recurrent cases. This was performed in order to rule out the influence of tumors that were aggressive to begin with. Age and sex of the patient had no association with recurrence status. Larger tumor size (categorized as ≤8 and >8 cm)11 was significantly associated with recurrence (P=0.031). Three of the eight patients with myasthenia gravis developed recurrence. Stage at diagnosis for 12 of the 18 patients with recurrence/metastases was stage I (four) or stage II (eight). There was no significant association between recurrence status and stage categorized as noninvasive (stage I) or invasive tumors (greater than stage I). As patients with advanced disease at presentation received chemotherapy/radiotherapy, the impact of these treatments on recurrence cannot be assessed in this study. Survival curves for patients with types A and AB thymomas, with and without recurrences, show a statistically significant difference (P=0.001 and 0.005, respectively) (Figure 2). Of the 18 patients with recurrence/metastases, 12 (67%) patients are alive, whereas six were lost to follow-up.
Discussion
The promulgated clinical behavior of types A and AB thymomas is not without controversy. Although some promote and anticipate a benign clinical course for these tumors,6, 12, 13, 14, 15, 16 others (ourselves included) consider all thymomas as potentially malignant neoplasms.1, 2, 10, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 Many prior studies have documented malignant behavior in types A and AB thymomas. In our retrospective study of 71 cases of types A and AB thymomas (37 cases with follow-up information available), 18 patients (nine each of types A and AB) developed recurrence and or metastases. These findings are in sharp contrast to the suggested benign course for patients with these subtypes.6, 12, 13, 14, 15, 16 Although our findings are subject to selection bias as patients with advanced/progressive disease are more likely to be referred for tertiary care, the study unequivocally shows the potential for aggressive behavior in these subtypes.
The classification of thymomas has been controversial and current nomenclature is based on a consensus building effort by Rosai et al.5 In the Muller-Hermelink–Kirchner (MHK) classification, types A and AB thymomas are designated as medullary and mixed types. In MHK's original series, none of these recurred or metastasized; although a single case of chest wall recurrence was noted.15 This case was considered the result of iatrogenic implantation metastasis because of a prior fine needle biopsy. The perception of benign behavior is aided by the slow growth of type A thymomas and that the majority is encapsulated or microinvasive (Masaoka stage 1)5, 6, 12 at the time of diagnosis. Moreover, the survival of patients with type A thymomas in some studies was 100% at 5 and 10 years15, 35 in spite of up to 20% of patients presenting with stage II or III disease.
The 2004 update of the 1999 WHO classification posits the Muller-Hermelink position and considers types A and AB as generally benign neoplasms.6 It states, ‘Generally, type A can be regarded as a benign tumor without having a risk of recurrence if the tumor can be completely removed surgically.’6 This statement is at odds with our findings and previous studies that have reported recurrences, metastases, and deaths2, 10, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 attributable to type A thymomas and cases classified as ‘spindle’ cell type in older series (Table 3 ). Recurrences and/or metastases have been described from 14 months to up to 20 years from diagnosis.21, 28 Similarly, we observed recurrences in follow-up from 6 months to 20 years (mean: 61.8 months) in nine of 20 patients with type A tumors. Blumberg et al17 reported a recurrence rate of 5% at 5 years, whereas in the series of Ciernik et al,20 two of the four patients with spindle cell tumors developed local recurrence. In majority of cases, relapses have been intrathoracic, although distant sites of progression include the lung, brain, liver, and thoracic spine.22, 29, 36 Importantly, in most of these studies, complete excision of the tumor was reported. Interestingly, in the study by Rieker et al,37 the 43 patients with type A tumors had a worse outcome when compared with patients with AB (n=20) and B1 (n=24) tumors. It is important for oncologists to be cognizant of the biologic potential of type A thymoma. With the continued perception of benign behavior, it is probable that some patients will not receive due consideration for adjuvant therapy.21, 38, 39
Regarding type AB thymoma, the 2004 WHO update states: ‘type AB thymomas are generally regarded as clinically benign tumors.’6 Paradoxically, it also states that up to 25% of AB thymomas can be invasive at diagnosis (stage II or greater).6 Their potential for recurrent behavior has been reported in several previous studies.19, 30, 35, 40, 41, 42, 43, 44 In our study, 16 of 29 patients had invasive tumors. In follow-up, we observed metastatic events from as early as 1 year to as late as 11 years (mean: 62.25 months) from diagnosis. Similarly, Park et al44 documented four recurrences in a 10-year follow-up period and three tumor-related deaths after complete excision. Studies have shown that patients with type AB thymoma are at an increased risk of relapse between 2 and 8 years (Table 4 ).30, 42 In some series, tumor recurrences were more frequent in type AB compared with type A thymoma.41, 44
Masaoka stage is a good predictor of aggressive behavior,10, 35, 38, 44 although recurrences have been documented even with early stage disease.10, 45, 46 The proportion of patients with recurrence was higher in stages III and IV compared with patients in stages I and II.38, 45 As most of the recurrent cases in this study were stage I or II at diagnosis, it was not useful in predicting the behavior of these tumors. In an analysis of 1320 thymic tumors, Kondo and Monden47 found total resection as the most significant predictive factor. However, patients with complete resection can still develop recurrences or die because of disease.30, 31, 39, 40 This has been suggested to occur more frequently with type AB than with type A tumors.44 In this study, the primary tumors in 17/18 patients had been completely excised, suggesting that complete resection is not curative in all patients with thymic neoplasms. Intraoperative adhesions have also been linked with the development of recurrence.26 It has been suggested that stage I tumors be further divided into stages Ia and Ib based on the absence or presence of peritumoral adhesions.21, 29 In some studies, recurrences were observed only among patients having intraoperative adhesions.21, 29
Patients with larger tumors appear to have worse prognoses.11, 17, 21, 48 In this study, tumors >8 cm had a significantly higher risk for recurrence. In contrast, Huang et al22 were not able to confirm the prognostic impact of the 8 cm cutoff; this may be due to the fact that there were very few large tumors in their series.
Progression of disease from low- to high-grade histology has been postulated as a cause of recurrence or metastases.49 In this study, two cases with matched primary and metastatic tumor slides were of identical histology. It is also possible that a more aggressive component could have been missed because of inadequate sampling. Indeed, in our series, we documented two cases with the presence of thymic carcinoma along with typical type A histology. However, these patients were lost to follow-up and prognostic impact of this feature could not be assessed. Lastly, as suggested by Suster and Moran,50 failure to recognize a spindle cell variant of thymic carcinoma could also explain the aggressive behavior.
The presence of myasthenia gravis as a prognostic parameter has been controversial. In the current series, 83% of the recurrent cases did not have myasthenia gravis. Similar results have been observed in prior studies, in which the better survival in patients with myasthenia had been attributed to earlier diagnosis.27, 51 However, the use of this factor in prognostication has been controversial as some studies have not found an association of survival with myasthenia gravis.29, 52, 53
In this current series, types A and AB thymomas had a similar pattern of recurrence/metastasis and thus can be regarded as a single category. Indeed, Suster and Moran54 have proposed a classification system that groups types A and AB along with types B1 and B2 under a single rubric of ‘typical thymoma.’ It is important to note that they recognize all thymic epithelial neoplasms as ‘potentially malignant.’ Thus, the term ‘benign thymoma’ for these subtypes should be discarded.7, 8, 29, 30 These tumors do exhibit tissue invasion and local recurrence even after complete resection. The data presented here and in Tables 3 and 4 clearly document their malignant potential.
The authors recognize certain inherent limitations and biases of this study. As patients included in this study were referred for specialized care, we were more likely to include advanced cases and the study population is, most likely, not representative of the prevalence of disease or its subtypes. The diagnosis of recurrent/metastatic disease was mainly based on clinical and imaging studies. For most cases, biopsy of the metastatic lesions was not performed; the reasons for doing so could include clinical presentation, the inaccessible location of lesions or the risk of damaging vital structures. Moreover, because of lack of complete surgical and clinical history, important associations of recurrent and/or metastatic disease with type of surgery and treatment regimens could not be made. Given the retrospective nature of the study and the selection biases, comments about treatment effect on survival cannot be made; these associations would be better tested in randomized clinical trials.
In summary, the study shows that WHO types A and AB thymomas are associated with recurrence/metastasis. Although, many patients with thymoma may develop recurrent disease several months to years from initial diagnosis, distant metastases affirm that all thymomas are to be considered malignant. Better stratification factors are needed to define strategies for optimizing treatment for thymoma patients. It is possible that a classification based on molecular features may define prognosis in patients with completely resected thymic tumors.
References
Levine GD, Rosai J . Thymic hyperplasia and neoplasia: a review of current concepts. Hum Pathol 1978;9:495–515.
Lattes R . Thymoma and other tumors of the thymus. An analysis of 107 cases. Cancer 1962;15:1224–1260.
Muller-Hermelink HK, Marino M, Palestro G, et al. Immunohistological evidences of cortical and medullary differentiation in thymoma. Virchows Arch A Pathol Anat Histopathol 1985;408:143–161.
Kirchner T, Muller-Hermelink HK . New approaches to the diagnosis of thymic epithelial tumors. Prog Surg Pathol 1989;10:167–189.
Rosai J . Histological Typing of Tumors of the Thymus 2nd edn, Vol. Springer-Verlag: Berlin, Germany, 1999.
Travis WD, Brambilla E, Muller-Hermelink HK, et al. Pathology and Genetics of the Lung, Pleura, Thymus and Heart, Vol., IARC Press: Lyon, France, 2004.
Suster S, Moran CA . Problem areas and inconsistencies in the WHO classification of thymoma. Semin Diagn Pathol 2005;22:188–197.
Detterbeck FC . Clinical value of the WHO classification system of thymoma. Ann Thorac Surg 2006;81:2328–2334.
Suster S, Moran CA . Histologic classification of thymoma: the World Health Organization and beyond. Hematol Oncol Clin North Am 2008;22:381–392.
Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981;48:2485–2492.
Wright CD, Wain JC, Wong DR, et al. Predictors of recurrence in thymic tumors: importance of invasion, World Health Organization histology, and size. J Thorac Cardiovasc Surg 2005;130:1413–1421.
Pan CC, Chen WY, Chiang H . Spindle cell and mixed spindle/lymphocytic thymomas: an integrated clinicopathologic and immunohistochemical study of 81 cases. Am J Surg Pathol 2001;25:111–120.
Venuta F, Rendina EA, Pescarmona EO, et al. Multimodality treatment of thymoma: a prospective study. Ann Thorac Surg 1997;64:1585–1591, discussion 91–2.
Harris NL, Muller-Hermelink HK . Thymoma classification. A siren's song of simplicity. Am J Clin Pathol 1999;112:299–303.
Marx A, Muller-Hermelink HK . Thymoma and thymic carcinoma. Am J Surg Pathol 1999;23:739–742.
Muller-Hermelink HK, Marx A . Thymoma. Curr Opin Oncol 2000;12:426–433.
Blumberg D, Port JL, Weksler B, et al. Thymoma: a multivariate analysis of factors predicting survival. Ann Thorac Surg 1995;60:908–913, discussion 14.
Chahinian AP, Bhardwaj S, Meyer RJ, et al. Treatment of invasive or metastatic thymoma: report of eleven cases. Cancer 1981;47:1752–1761.
Chalabreysse L, Roy P, Cordier JF, et al. Correlation of the WHO schema for the classification of thymic epithelial neoplasms with prognosis: a retrospective study of 90 tumors. Am J Surg Pathol 2002;26:1605–1611.
Ciernik IF, Meier U, Lutolf UM . Prognostic factors and outcome of incompletely resected invasive thymoma following radiation therapy. J Clin Oncol 1994;12:1484–1490.
Gamboa EO, Sawhney V, Lanoy RS, et al. Widespread metastases after resection of noninvasive thymoma. J Clin Oncol 2008;26:1752–1755.
Huang J, Rizk NP, Travis WD, et al. Comparison of patterns of relapse in thymic carcinoma and thymoma. J Thorac Cardiovasc Surg 2009;138:26–31.
Ibrahim NB, Briggs JC, Jeyasingham K, et al. Metastasising thymoma. Thorax 1982;37:771–773.
Koga K, Matsuno Y, Noguchi M, et al. A review of 79 thymomas: modification of staging system and reappraisal of conventional division into invasive and non-invasive thymoma. Pathol Int 1994;44:359–367.
LeGolvan DP, Abell MR . Thymomas. Cancer 1977;39:2142–2157.
Lewis JE, Wick MR, Scheithauer BW, et al. Thymoma. A clinicopathologic review. Cancer 1987;60:2727–2743.
Monden Y, Nakahara K, Iioka S, et al. Recurrence of thymoma: clinicopathological features, therapy, and prognosis. Ann Thorac Surg 1985;39:165–169.
Naniwa T, Kakihara H, Zen-nami S, et al. [Recurrence of thymoma accompanied with hypogammaglobulinemia 20 years after surgery: a case report]. Nihon Kokyuki Gakkai Zasshi 2002;40:241–244.
Regnard JF, Magdeleinat P, Dromer C, et al. Prognostic factors and long-term results after thymoma resection: a series of 307 patients. J Thorac Cardiovasc Surg 1996;112:376–384.
Rena O, Papalia E, Maggi G, et al. World Health Organization histologic classification: an independent prognostic factor in resected thymomas. Lung Cancer 2005;50:59–66.
Singhal S, Shrager JB, Rosenthal DI, et al. Comparison of stages I-II thymoma treated by complete resection with or without adjuvant radiation. Ann Thorac Surg 2003;76:1635–1641, discussion 41–2.
Tan PH, Sng IT . Thymoma—a study of 60 cases in Singapore. Histopathology 1995;26:509–518.
Verley JM, Hollmann KH . Thymoma. A comparative study of clinical stages, histologic features, and survival in 200 cases. Cancer 1985;55:1074–1086.
Wang LS, Huang MH, Lin TS, et al. Malignant thymoma. Cancer 1992;70:443–450.
Okumura M, Ohta M, Tateyama H, et al. The World Health Organization histologic classification system reflects the oncologic behavior of thymoma: a clinical study of 273 patients. Cancer 2002;94:624–632.
Guillan RA, Zelman S, Smalley RL, et al. Malignant thymoma associated with myasthenia gravis, and evidence of extrathoracic metastases. An analysis of published cases and report of a case. Cancer 1971;27:823–830.
Rieker RJ, Hoegel J, Morresi-Hauf A, et al. Histologic classification of thymic epithelial tumors: comparison of established classification schemes. Int J Cancer 2002;98:900–906.
Chen G, Marx A, Wen-Hu C, et al. New WHO histologic classification predicts prognosis of thymic epithelial tumors: a clinicopathologic study of 200 thymoma cases from China. Cancer 2002;95:420–429.
Lardinois D, Rechsteiner R, Lang RH, et al. Prognostic relevance of Masaoka and Muller-Hermelink classification in patients with thymic tumors. Ann Thorac Surg 2000;69:1550–1555.
Kim DJ, Yang WI, Choi SS, et al. Prognostic and clinical relevance of the World Health Organization schema for the classification of thymic epithelial tumors: a clinicopathologic study of 108 patients and literature review. Chest 2005;127:755–761.
Mineo TC, Ambrogi V, Mineo D, et al. Long-term disease-free survival of patients with radically resected thymomas: relevance of cell-cycle protein expression. Cancer 2005;104:2063–2071.
Okumura M, Miyoshi S, Fujii Y, et al. Clinical and functional significance of WHO classification on human thymic epithelial neoplasms: a study of 146 consecutive tumors. Am J Surg Pathol 2001;25:103–110.
Okumura M, Shiono H, Inoue M, et al. Outcome of surgical treatment for recurrent thymic epithelial tumors with reference to world health organization histologic classification system. J Surg Oncol 2007;95:40–44.
Park MS, Chung KY, Kim KD, et al. Prognosis of thymic epithelial tumors according to the new World Health Organization histologic classification. Ann Thorac Surg 2004;78:992–997, discussion 7–8.
Rea F, Marulli G, Girardi R, et al. Long-term survival and prognostic factors in thymic epithelial tumours. Eur J Cardiothorac Surg 2004;26:412–418.
Marchevsky AM, Gupta R, McKenna RJ, et al. Evidence-based pathology and the pathologic evaluation of thymomas: the World Health Organization classification can be simplified into only 3 categories other than thymic carcinoma. Cancer 2008;112:2780–2788.
Kondo K, Monden Y . Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878–884, discussion 84–5.
Nakagawa K, Asamura H, Matsuno Y, et al. Thymoma: a clinicopathologic study based on the new World Health Organization classification. J Thorac Cardiovasc Surg 2003;126:1134–1140.
Kuo TT, Chan JK . Thymic carcinoma arising in thymoma is associated with alterations in immunohistochemical profile. Am J Surg Pathol 1998;22:1474–1481.
Suster S, Moran CA . Spindle cell thymic carcinoma: clinicopathologic and immunohistochemical study of a distinctive variant of primary thymic epithelial neoplasm. Am J Surg Pathol 1999;23:691–700.
Maggi G, Casadio C, Cavallo A, et al. Thymoma: results of 241 operated cases. Ann Thorac Surg 1991;51:152–156.
Pescarmona E, Rendina EA, Venuta F, et al. Analysis of prognostic factors and clinicopathological staging of thymoma. Ann Thorac Surg 1990;50:534–538.
Quintanilla-Martinez L, Wilkins Jr EW, Choi N, et al. Thymoma. Histologic subclassification is an independent prognostic factor. Cancer 1994;74:606–617.
Suster S, Moran CA . Thymoma, atypical thymoma, and thymic carcinoma. A novel conceptual approach to the classification of thymic epithelial neoplasms. Am J Clin Pathol 1999;111:826–833.
Acknowledgements
We thank Tracey Bender for proofreading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Modern Pathology website
Supplementary information
Rights and permissions
About this article
Cite this article
Jain, R., Mehta, R., Henley, J. et al. WHO types A and AB thymomas: not always benign. Mod Pathol 23, 1641–1649 (2010). https://doi.org/10.1038/modpathol.2010.172
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2010.172
Keywords
This article is cited by
-
A large microRNA cluster on chromosome 19 is a transcriptional hallmark of WHO type A and AB thymomas
British Journal of Cancer (2016)
-
Solitary metastasis to the breast after complete resection of encapsulated type AB thymoma: a case report
Journal of Medical Case Reports (2015)
-
Micronodular thymic neoplasms: case series and literature review with emphasis on the spectrum of differentiation
Modern Pathology (2015)
-
The prognostic value of architectural patterns in a study of 37 type AB thymomas
Modern Pathology (2014)
-
The role of histology in predicting recurrence of type A thymomas: a clinicopathologic correlation of 23 cases
Modern Pathology (2013)