Abstract
Thymomas are rare tumors characterized by a broad range of morphologic appearances that can sometimes give rise to difficulties for classification. We have studied a series of 120 thymoma patients in whom the tumors were characterized by sheets of atypical epithelial cells with squamoid and/or spindle cell features. They occurred in 63 men and 57 women and presented as a discrete mass in the anterior mediastinum measuring 2–23 cm (mean: 8.2 cm). Patients’ ages ranged from 14 to 86 years (mean: 57.8) and most had symptoms referable to a mass lesion. 20 patients had myasthenia gravis or other autoimmune disorder. 76 cases were characterized by a predominant population of round to polygonal tumor cells while 32 cases were characterized by atypical oval or spindle cells. 12 cases showed mixed features and 16 cases showed the development of thymic carcinoma arising from thymoma. All cases were positive for p40/p63 and cytokeratin AE1/AE3. 23 cases were positive for CD5 (25%), and 13 for CD117 (14%). MIB1 showed a significant increase in proliferative activity (mean = 11.6%). Next generation sequencing in 47 cases did not disclose any variants amenable to current targeted therapies. Clinical follow up ranging from 2 to 29 years showed a progressive increase in aggressive behavior and fatality rate with advancing stage. Overall survival was 87% at 5 years, 67% at 10 years, and 23% at 20 years. Completeness of resection and staging were the most significant parameters for survival. The more aggressive tumors followed a protracted clinical course with multiple recurrences and metastases over a long period of time (mean = 19.8 years from time of initial relapse to death). Atypical thymomas are a distinct category of thymic epithelial neoplasm characterized by a slowly progressive clinical course with increased potential for metastases, transformation to a higher-grade malignancy, and fatal outcome in some cases.
Similar content being viewed by others
Introduction
Thymomas represent the most common type of primary thymic epithelial neoplasm. Due to their variegated morphologic appearances, histopathologic classification of thymoma has been a topic of debate for many years with various authors favoring either splitting or lumping of the various categories. The current World Health Organization (WHO) histologic classification of thymoma1 follows a format initially proposed by Rosai in 19992, who classified these tumors based on their cell type (round vs. spindle) and their proportion of background lymphocytes into five categories designated as type A, AB, and B, with the B category being further split into types B1, B2 and B3 based on the relative proportion of lymphocytes to epithelial cells and the increase in cytologic atypia of the neoplastic cells. While the diagnostic criteria for the WHO classification have been refined over the past few years, some outstanding issues remain, particularly when making the distinction between B2 and B3 thymoma and between B3 and thymic carcinoma, as well as for distinguishing the new WHO category of “atypical type A thymoma” from B3 thymomas with spindle cell morphology1.
We have studied a series of 120 thymoma patients in whom the tumors were characterized histologically by increased cytologic atypia in comparison with the more conventional types of thymoma (WHO types A, AB, B1 and B2). A clinicopathologic, immunohistochemical, and molecular genetic study of these cases is presented along with their long-term clinical follow-up to further delineate the clinicopathologic features of these tumors. A discussion of the literature on the topic is presented.
Materials and methods
Case selection
Cases diagnosed as epithelial, epithelial-rich, atypical thymoma and WHO type B3 thymoma were retrieved from the surgical pathology files of the Beth Israel Deaconess Medical Center, Boston, MA (1990–2016), the Medical College of Wisconsin, Milwaukee, WI (1987–2016) and from the personal consultation files of one of the authors (SS). 120 cases containing adequate histopathologic diagnostic material and bearing the features here described were selected for the study. From 6 to 25 glass slides were reviewed in all cases. All the cases included in this study corresponded to surgical resection specimens; tumors in which only a biopsy was available for evaluation were excluded from the study. On histologic examination, a few cases showed admixtures and transitions with other types of thymoma (i.e., type A, AB, B1 or B2); only cases in which the atypical components accounted for >50% of the lesion and represented the majority of the tumor were included in the study. The histologic inclusion criteria for the cases in the study were tumors showing a sheet-like growth pattern with cells touching each other, paucity of lymphocytes, polygonal to oval or spindle cells with increased nuclear to cytoplasmic ratios, dense chromatin pattern and nucleoli, and scattered mitotic figures. The information abstracted from the records included: age, sex, clinical symptoms and associated conditions, size of the tumor and location, extent of the resection, status of the margins, clinical staging according to the modified Masaoka scheme proposed by Koga et al.3, histopathologic features and clinical follow up. Clinical follow-up was obtained from the hospital’s medical records or by contacting the referring physician. The study was conducted under appropriate institutional review board approval from both institutions.
Immunohistochemical studies
For immunohistochemical studies, a tissue microarray (TMA) was prepared for the in-house cases. Immunohistochemical stains were performed on the consultation cases from representative unstained slides or from whole tissue sections from submitted paraffin blocks. 42 cases were included in the TMA and 50 cases were evaluated on whole slide tissue sections.
For the creation of the TMA, slides stained with hematoxylin and eosin were reviewed for each case and used to mark representative areas of the tumor from each case. 1 mm tissue cores containing tumor tissue were taken from paraffin-embedded samples and arrayed using a Veridiam Tissue Arrayer (Oceanside, CA). Three cores per case were placed onto the TMA. After the cores were placed, TMA blocks were heated in an oven at 37 °C for 1 h and cooled at room temperature. Sections from the tissue microarray were cut at 4 µm and used for subsequent immunohistochemical analysis. Immunohistochemical studies were performed on all cases using a standard technique; 4 µm thick sections were cut and deparaffinized in xylene, hydrated in descending dilutions of ethanol, and exposed to heat-induced epitope retrieval. Immunohistochemical staining was performed using reagents from the Dako Envision FLEX kit and the Dako AutostainerPlus stainer (Agilent, Santa Clara, CA). The antibodies tested included cytokeratin AE1/AE3, p40, p63, PAX8, chromogranin, synaptophysin, CD56, bcl-2, MIB1, e-cadherin, beta-catenin, calretinin, EMA, CD3, CD5, CD20, CD45, CD117 and TdT; sources and dilutions are presented in supplementary Table 1. Following pretreatment with Target Retrieval Solution, tissue was blocked with peroxidase-blocking reagent for 5 min and incubated with the primary antibody at room temperature. Signals were detected using the Dako FLEX detection kit. Counterstaining was performed with Envision FLEX hematoxylin for 7 min at room temperature. Appropriate positive and negative controls were run concurrently for all antibodies tested. The immunohistochemical reaction was graded as positive based on nuclear, cytoplasmic or membrane reactivity for the various antibodies. Positivity was regarded as significant when it involved the neoplastic epithelial cells and affected at least 50% of the tumor cell population. For the evaluation of proliferative activity, a multiplex cocktail composed of MIB-1/CD45 antibodies was prepared to permit adequate separation of lymphocytes from the neoplastic epithelial cells. Labeling index for MIB-1 was determined by counting 100 tumor cells using a ×60 high-dry objective with a ×22 eyepiece, and the results were expressed as a percentage of positive cells.
Statistical analysis
Statistical analysis was performed using the R software environment4. Age at resection was assessed with respect to recurrence using logistic regression. Predictive pathologic features, including gross extent of resection, clinical stage, status of the margins, histologic type, vascular invasion, cystic and hemorrhagic changes, presence of other type of thymoma or thymic carcinoma, and CD5/CD117 immunostaining were evaluated with respect to recurrence and metastases by univariate analysis (Fisher exact test) and multivariate analysis (logistic regression). Survival analysis (overall survival and recurrence-free survival) was evaluated using Kaplan–Meir plots and by performing log-rank tests.
Molecular analysis
Forty-seven cases were analyzed using the Illumina Cancer Hotspot Panel v2 (product number 20019161) using paired end 2 × 300 read format with average 500× depth per sample. Representative paraffin blocks were selected from the cases and unstained slides were cut, representative tissue was manually dissected, and highly purified DNA was extracted. DNA and library concentrations were quantified using a Tape Station (Agilent), spectrofluorometry (Qubit), and NanoDrop (Thermo Fisher). The purified DNA was used to prepare a library following the AmpliSeq for Illumina Cancer Hotspot Panel v2 protocol (207 primers pairs/pool). Libraries were normalized, pooled, and then loaded onto a flow cell for sequencing. Data was processed with the Local Run Manager to perform alignment and variant calling. VCF files were annotated using Annovar based on the hg19 reference genome. Each position in the VCF files was annotated by the gene name, type of mutation, transcript, and protein information. All figures and statistical calculations were performed using R statistical software (ver. 3.6.1; https://www.r-project.org/)5.
Results
Clinical findings
The clinical features of our patients are detailed in Table 1. Patients’ ages ranged from 14 to 86 years (mean: 57.8); 57 were women and 63 were men. 108 patients presented with symptoms attributable to a mass lesion in the chest cavity, including chest pain, shortness of breath and pleural effusion; 12 were asymptomatic and discovered on routine radiographic examination for other causes or were found incidentally during coronary artery bypass graft surgery. Four cases with advanced stage tumors presented with superior vena cava syndrome. 18 patients (15%) were diagnosed with myasthenia gravis at the time the mass was discovered; one of them also had concurrent aplastic anemia. One patient had agammaglobulinemia (Good syndrome), and another patient had aplastic anemia. 16 patients had concurrent or synchronous malignancies, including vulvar, endometrial, colon, prostate, breast, lung, salivary gland, and thyroid carcinoma; one patient developed chronic myelomonocytic leukemia, and one patient subsequently developed a diffuse large B-cell lymphoma. Two patients had synchronous benign tumors, including meningioma and giant cell tumor of bone. All patients underwent surgical excision; in 14 patients the tumors were unresectable and a debulking procedure with subtotal resection was performed; in the remainder a complete thymectomy by either transsternal approach (106 cases) or video-assisted thoracoscopic surgery (14 cases) was done. 51 patients were treated with postoperative radiation to the chest cavity, and 17 patients also received chemotherapy, either preoperative or postoperative (Table 1). Local recurrences were documented in 22 patients and distant metastases in 25 patients. 24 patients underwent re-excision for recurrent or metastatic tumors. Time from initial surgery to local recurrence ranged from 1 to 23 years (mean: 6.4 years). Time from initial surgery to the appearance of metastatic disease ranged from 1 to 11 years (mean: 4.7); two patients presented with distant metastases at the time of initial diagnosis. 42 patients were alive with no evidence of disease at last follow-up; 7 were alive with disease between 5 and 38 years (mean: 16.2 years), 17 died of tumor, and 2 died in the immediate postoperative period of complications of surgery. The most common sites of metastases were the pleura (15 cases) and lungs (13 cases), followed by ribs/chest wall (8) and liver (6). Other less common sites of metastases included the pericardium, brain, and diaphragm (3 each), adrenals, skeletal muscle, and spine (2 each), and spleen and kidney (1 each). 12 patients died of unrelated causes, including secondary malignancies (renal cell carcinoma, endometrial carcinoma, T-cell lymphoma), aplastic anemia, heart failure, or unknown causes.
Pathologic findings
Atypical thymoma with squamoid features
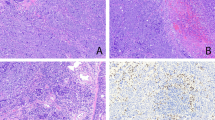
76 cases in the study were characterized by a cohesive population of round to polygonal tumor cells with enlarged nuclei surrounded by abundant eosinophilic cytoplasm. These cases represent the equivalent of the tumors currently designated as type B3 thymoma in the WHO classification1. The most distinctive diagnostic feature for these tumors was their sheet-like growth pattern, unlike the type B1 and B2 tumors in the WHO classification in which the proliferating neoplastic cells are non-cohesive and are mostly found singly scattered or forming minute clusters surrounded by numerous lymphocytes. The tumors displayed various growth patterns on scanning magnification, including a lobulated, multinodular growth pattern separated by fibrous bands (Fig. 1A) and a solid sheet-like growth pattern with numerous dilated perivascular spaces (Fig. 1B). Some cases showed a striking tendency for palisading of the tumor cells around the dilated perivascular spaces; in a few cases the perivascular spaces also displayed perivascular hyalinization. The stroma of the tumor was mostly fibrous with occasional foci of calcification; two cases also contained metaplastic bone. In the invasive cases, the stroma of the tumor at the advancing edges was intensely desmoplastic and harbored scattered small islands of tumor cells. In 17 cases, prominent cystic and hemorrhagic changes with extensive areas of necrosis and infarction were present. The areas of necrosis and infarction were associated with thrombotic events in the adjacent stroma. Minute foci of coagulative tumor cell necrosis unassociated with cystic and hemorrhagic changes were also found in 2 cases. The cytologic composition of the tumors was variable; the size of the tumor cells ranged from small and round (the size of a histiocyte), to larger polygonal cells (2–3 times the size of a normal histiocyte), to scattered pleomorphic cells with anaplastic nuclei (4–5 times the size of a normal histiocyte). Most of the tumor cells were characterized by a high nuclear to cytoplasmic ratio and showed enlarged hyperchromatic nuclei surrounded by abundant eosinophilic cytoplasm (Fig. 2A); the dense nuclear chromatin observed in these cells contrasted with the pale nuclei with lightly scattered heterochromatin normally seen in types B1 and B2 thymoma. Nucleoli were also present and often prominent in the tumor cells, particularly in the cells that were larger than surrounding cells (Fig. 2B). Some of the nuclei displayed unusual but distinctive features, such as complex infoldings and irregularities of the nuclear envelope resulting in a “raisinoid” or convoluted appearance; in others cases the tumor cells displayed prominent longitudinal nuclear grooves. Cells containing nuclei that showed an elongated, spindled appearance could also be found scattered among the epithelioid cell population. Focal areas displaying striking cytologic atypia with marked anaplasia, intranuclear pseudoinclusions and multinucleated or multilobated nuclei were also seen in 2 cases (cases 58 and 97) (Fig. 2C); these were felt to be the result of degenerative changes, possibly related to prior radiation or chemotherapy. Mitotic activity was generally low and ranged from 0 to 12 per 10 high power fields (median: 1.75). Abnormal tripolar mitoses could also be occasionally found. The cytoplasm of the tumor cells in all cases was abundant and densely eosinophilic. A highly distinctive feature of these tumors was the thick, sharply delineated cell membranes lacking intercellular bridges that surrounded the tumor cells resulting in a pavement-like architecture closely reminiscent of squamous epithelium (i.e., “squamoid appearance”) (Fig. 3A). Some cases showed a clear cytoplasmic perinuclear halo while a few cases displayed complete clearing of the cytoplasm imparting the tumor with a striking “clear cell” appearance (Fig. 3B). Another distinctive feature seen in 12 cases were scattered foci of abrupt keratinization; the cells within these areas were tightly packed and sharply demarcated from the surrounding cell population and showed closely apposed cell membranes displaying a pavement-like architecture with occasional intercellular bridges, single cell keratinization and scattered keratohyaline granules (Fig. 3C). Confluence of the foci of abrupt keratinization was seen in 6 cases creating islands that resembled immature squamous epithelium; however, small irregular and infiltrative islands of squamous epithelium with marked cytologic atypia and increased mitotic activity, as seen in well-differentiated squamous cell carcinoma, was not a feature seen in any of these tumors. Areas displaying transitions with other types of thymoma were present in 30 cases; in all those cases the atypical thymoma component accounted for the majority of the tumor. Gradual transitions between the squamoid atypical component and more conventional areas of B1 or B2 thymoma were seen in 28 cases; the most common combination was atypical thymoma with B2 thymoma (25 cases), followed by atypical thymoma with B1 thymoma (3 cases) and one case containing both areas of B1 and B2 thymoma (Fig. 4A). In two cases, areas of atypical thymoma in association with spindle cell thymoma (WHO type A) were seen; in 2 cases atypical thymoma was seen to merge with a lymphocyte-rich spindle cell thymoma (WHO type AB), and in 2 cases, a combination of atypical thymoma with WHO type B2 and type A was seen. In 8 cases, the tumors showed the emergence of a higher-grade lesion with features of thymic carcinoma arising from the background of atypical thymoma (Fig. 4B). In 7 cases, the thymic carcinoma component showed features of poorly differentiated non-keratinizing squamous cell carcinoma and in one case the tumor showed features of spindle cell thymic carcinoma. The areas of carcinoma showed marked cytologic atypia, increased mitotic activity and tumor cell necrosis, and appeared to gradually blend and transition with the underlying thymoma.
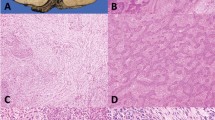
A Nuclei of varying sizes show dense chromatin pattern with ample rim of cytoplasm; notice longitudinal grooves in large cell in the center of the field; B Atypical cell with enlarged nucleus, dense chromatin and prominent nucleolus is seen in the center of the field; C Scattered cells are seen displaying striking nuclear pleomorphism but without mitotic activity, possibly related to prior radiation.
A Sheets of large epithelioid cells displaying sharp cell membranes and a pavement-like architecture; notice several crinkled, “raisinoid” nuclei; B Tumor cells with abundant clear cytoplasm. Notice the dense hyperchromatic nuclei with irregular nuclear contours and sharply delineated cell membranes characteristic of these tumors; C Foci of squamous differentiation are seen in this tumor; the cells however do not display significant atypia or mitotic activity to qualify for squamous cell carcinoma.
A Transitions between atypical thymoma (left) and lymphocyte-rich thymoma (WHO B1) (right); B Transitions between atypical thymoma and poorly differentiated, non-keratinizing squamous cell thymic carcinoma. Small residual islands of atypical thymoma are seen gradually merging with the poorly differentiated, infiltrative components.
Atypical thymoma with spindle cell features
32 cases showed a predominantly spindle cell morphology and were characterized by sheets of enlarged and hyperchromatic spindle cells with oval nuclei that showed prominent nucleoli and scattered mitoses (Fig. 5A). The spindle cells contained abundant cytoplasm but did not display the sharply outlined cells membranes seen in the squamoid tumors. Mitotic counts ranged from 0 to 12 per 10 high power fields and were on average higher than in the squamoid tumors (mean: 2.48 per 10 high power fields) (Fig. 5B). Dilated perivascular spaces with palisading of tumor cells around the lumen were also present. 8 cases were found to be associated with an underlying spindle cell thymoma (WHO type A) and 6 with a lymphocyte-rich spindle cell thymoma (WHO type AB); in 3 cases, the tumors were seen to arise from preexisting micronodular thymomas with B-cell hyperplasia. In all these cases, the atypical thymoma component predominated and accounted for >50% of the lesion. One case showed a combination of spindle cell atypical thymoma with areas of WHO type B1 and B2 thymoma. Foci of abrupt keratinization were observed in 3 cases, and areas with cystic and hemorrhagic changes with extensive necrosis and infarction were present in 5. Pinpoint microscopic areas of coagulative necrosis were seen in one case. In 8 cases, the tumors showed foci of high-grade transformation to thymic carcinoma. In 5 cases, the higher-grade areas had features of poorly differentiated non-keratinizing squamous cell carcinoma; and in one case each the carcinomatous component showed features of spindle cell thymic carcinoma, basaloid carcinoma, and anaplastic carcinoma.
Atypical thymoma with mixed features
In 12 cases, the tumors showed an approximately equal admixture of areas demonstrating both spindle cell and squamoid features. Two of these cases showed focal transitions with WHO type A thymoma and 1 case with WHO type B2 thymoma. 2 cases showed foci of abrupt keratinization and 3 cases showed areas of cystic and hemorrhagic changes with extensive necrosis and infarction.
Immunohistochemical findings
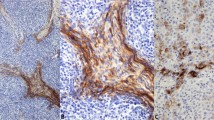
Representative material was available for immunohistochemical studies in 92 cases. All cases in the study showed strong nuclear positivity of the tumor cells for p40 and p63, and strong cytoplasmic staining for cytokeratin AE1/AE3. In 60/92 cases (65%), PAX8 showed diffuse nuclear staining in most of the tumor cells like that seen for p40/p63. 12/92 (13%) cases showed only weak, patchy and focal positivity for PAX8 in scattered cells. EMA was focally positive in 18/92 (20%) cases but only in the small areas of abrupt keratinization; in 3 cases, however, EMA also stained the sheets of polygonal tumor cells unassociated with the foci of abrupt keratinization. Chromogranin and synaptophysin were convincingly positive in only one case. Calretinin was negative in all cases. The lymphoid cell component strongly labeled with CD3, TdT and bcl-2 in all cases. CD20 showed a few scattered positive lymphocytes around dilated perivascular spaces and was also strongly positive in the lymphocytes in the stroma and lymphoid follicles of the cases that harbored a micronodular thymoma component. Bcl-2 also showed cytoplasmic staining of the epithelial tumor cells in 22/32 (69%) cases of atypical thymoma with spindle cell features and in the spindle cell component in 6/12 (50%) cases of mixed squamoid/spindle cell atypical thymoma. CD5 was positive in the epithelial tumor cells in 23/92 cases (25%); the staining pattern was diffuse membranous, or cytoplasmic and membranous (Fig. 6A). CD117 was positive in the epithelial tumor cells in 13/92 cases (14%); the staining pattern was mainly membranous and diffuse (Fig. 6B). CD117 was also positive in the thymic carcinoma component in 14/16 (88%) cases. Distribution of the staining patterns for CD5 and CD117 in our cases is shown in Table 2. MIB-1 was assessed using a cocktail of MIB-1/CD45 to distinguish the epithelial cells from lymphocytes (Fig. 6C). Overall percentage of MIB-1 positivity in the entire cohort was 11.6%. Table 2 also shows the distribution of the percentages of positivity for MIB1 by histologic type, staging and outcome. The spindle cell type of atypical thymoma showed a slightly higher percentage of nuclear positivity than the squamoid type (13.66% vs. 11.85%). There was also a minimal trend for slightly higher proliferative activity found in higher stages (III–IV) than in the lower stages (I–II). Patients with recurrences and metastases, and those who died of tumor also showed a trend for an increase in the proliferation rate (14.25%, 15.94%, and 16.43%, respectively), but the differences from the mean were minimal. It should be noted, however, that there was wide variability in proliferative activity ranging from 0 to 50% nuclear positivity; however, cases with very low or very high proliferative activity were in the minority and >80% of cases were in the range of 8–20%.
A Immunohistochemical staining for CD5 in atypical thymoma shows strong membrane positivity; notice positive staining in scattered small T-lymphocytes in the stroma; B Immunohistochemical staining for CD117 shows strong membrane staining for this antigen in the tumor cells; C Multiplex staining for Ki-67 and CD45 in atypical thymoma shows increased proliferation rate with ~15% positive nuclei (brown chromogen); notice scattered small lymphocytes (red chromogen) in the background.
Next generation sequencing (NGS)
In total, 20 unique, non-synonymous variants were identified in the cohort of 47 cases. In these cases, between 1 and 4 non-synonymous variants were identified: 19 cases with 1 variant, 15 cases with 2, 10 cases with 3, and 4 cases with 4 variants (Fig. 7). The deleteriousness of each variant was assigned using combined annotation-dependent depletion (CADD) score, which is used to prioritize causal variants6. The primary use of CADD is to identify and score variants that are most likely to be deleterious and potentially pathogenic. Higher CADD scores indicate that a variant is more likely to have deleterious effects and thus CADD scores are used to prioritize variants from among a cadre of candidates. In our cohort, 7 variants were identified in more than one case. Of these only RET p.A641S, identified in 3 patients (6.3%), is predicted as likely pathogenic. None of the variants identified could be targeted by specific therapies.
Samples are shown in columns and variations are shown in rows. For each variation, the gene name and the amino acid affected for the major transcript are indicated on the right. For each sample, each variation was given a value from 0, 1, or 2, based on whether the variant is absent, heterozygous, or homozygous, respectively. The variants are sorted from top to bottom from high (most deleterious) to low (least deleterious) CADD scores.
Statistical analysis
Logistic regression analysis for recurrence or metastasis by patient age at the time of surgery did not reveal any significant associations. On univariate analysis for recurrence, only low clinical stage (p < 0.0001) and negative pathologic margin status (p < 0.0001) were found to be statistically significant. Assessment of resection status (complete vs. incomplete resection) showed a trend toward significance (p = 0.1). Features associated with metastasis included low clinical stage (p < 0.0001), negative pathologic margins (p < 0.0001) and complete resection (p < 0.0001). Histologic type (epithelioid, spindle or mixed) did not show any association with recurrence. Spindled morphology, however, was associated with a decreased tendency for metastases (4/18 cases compared with 18/51 for squamoid and 3/10 for mixed; p = 0.3 on Fisher’s exact test). Presence of vascular invasion, cystic and hemorrhagic changes, other type of thymoma or thymic carcinoma component, and CD5/CD117 positivity did not show any significant association with recurrence or metastasis. On multivariate analysis, only positive margins showed a significant association with recurrence (p < 0.001), and both the status of the margins and histologic type showed significant association with metastases. Overall, 5-, 10- and 20 years survival rates were 87%, 67%, and 23%, respectively in the censored data set. The 5- and 10 years survival rates in patients aged <50 years were like those for patients >50 (85% and 66% vs. 88% and 61%). Comparison of survival probability in patients with an atypical thymoma (with or without transformation to thymic carcinoma) as the only known neoplasm versus patients with atypical thymoma (+/− thymic carcinoma) who harbored a concurrent extrathymic malignancy showed that the occurrence of extrathymic tumors was associated with a significant decrease in overall survival (p = 0.05, log-rank test) (Supplemental Fig. 1). Among the clinicopathologic features studied, recurrence-free and overall survival were found to be related only to resection status (p = 0.00001, log-rank test), margin status (p = 0.05), and clinical stage (p = 0.01) (Fig. 8). The 10-year overall survival of patients with negative pathologic margins was better than for those with positive margins (84% vs. 57%; log-rank p < 0.001). The 10-year overall survival of patients with stage I and II disease were better than those with stages III and IV (87% vs. 52%, log-rank p < 0.001). The other pathologic parameters did not predict significant differences in recurrence-free or overall survival.
A Increased probability of longer recurrence-free survival time was seen for cases with compete resection compared with subtotal resection (p = 1e−05 by log-rank test; Chisq = 19.3; n = 78, 42 censored); B Overall survival in these groups showed a trend towards improvement with complete resection but did not achieve statistical significance (p = 0.08, Chisq = 3.1). C Increased probabilities of longer recurrence-free survival (p = 3e−05 log rank, Chisq = 17.7; n = 76; 44 censored) and D overall survival (p = 0.05; Chisq = 3.7) were found for cases with negative margins, compared with those with positive margins. E Clinical stage was also found to be a predictor of recurrence-free survival (p = 4e−10 log rank; Chisq = 52.6 (5 d.o.f); n = 77; 43 censored) and F overall survival (p = 0.01 log rank; Chisq = 14.5 (5 d.o.f.). The largest difference in survival probability was seen between clinical stages IIb and III.
Discussion
The classification of thymoma has remained problematic over the years and continues to be a work in-progress7. In-keeping with established tradition, pathologists have attempted to correlate the histology of thymoma with their clinical behavior to offer prognostic guidance for the treating physician. Given the wide spectrum of morphologic appearances displayed by these tumors this has remained a challenging task. In 1999, Dr. Rosai and a select panel of experts sponsored by the WHO proposed a new approach for the classification of these tumors that substituted the previous terminologies for a schema composed of a combination of letters and numbers2. This schema was, by the authors’ own admission, introduced simply as a way to facilitate translation between the various existing classifications and not as a definitive histological classification; however, subsequent literature supporting the purported validity of this schema led to its adoption as a substitute for a more scientifically based histologic classification. The current WHO approach, however, simply represents the continuation of the older traditional morphologic classifications proposed more than 50 years earlier by Lattes8 and Bernatz et al.9, classifications that were also based on the shape of the proliferating tumor cells (spindle vs. round/epithelioid) and the varying proportion of accompanying lymphocytes. Of the various histologic types of thymoma, the WHO type B3 thymoma seems to be the most poorly understood and has been repeatedly cited as posing problems for differential diagnosis with B2 thymoma and thymic carcinoma10,11,12,13,14. Many studies have been published in the literature attempting to establish a correlation between the various WHO categories and prognosis15,16,17,18,19. The majority of the larger studies, however, appear to indicate that staging, not histology, is the most significant parameter for the prognostication of these tumors20,21,22,23,24,25,26. In a large study from the European Society of Thoracic Surgeons database involving 2030 patients undergoing surgery for thymoma, statistical analysis revealed that the most significant prognostic parameters for overall survival were modified Masaoka staging and completeness of resection; the prognostic significance of histology in that series could not be demonstrated25. The largest retrospective review of thymomas to date from the International Thymic Malignancy Interest Group (ITMIG) database also showed on multivariate analysis that stage and resection status had the greatest impact on survival and recurrence while histology seemed to have only minor impact on recurrence22; the study, however, suffered the limitation that central review of the histology for the cases was not performed.
We have studied a series of 120 cases of thymoma with a focus on cases displaying atypical histologic features to better define their clinicopathologic, immunohistochemical and molecular characteristics and correlate them with clinical behavior. Tumors with such features have been variously designated in the past as epithelial-rich thymoma, polygonal cell thymoma, and well-differentiated thymic carcinoma7,8,9,26,27,28. These tumors roughly correspond in the current WHO classification1 to thymoma type B3 and have also been designated by us previously as “atypical thymoma” due to the obvious increase in cytologic atypia observed in comparison with the other histologic types29. We believe these tumors correspond to a more advanced stage in the progression of thymic malignancy and occupy an intermediate position (i.e., equivalent to moderately differentiated tumors) in the spectrum of neoplastic progression of thymic epithelial neoplasms29,30. Although this type of thymoma has been included in a few large retrospective series of thymoma23,24,25, comprehensive clinicopathologic studies specifically addressing these tumors have not yet been presented.
The current study highlights the broad spectrum of morphologic appearances that can be displayed by thymomas characterized by cytologic atypia, including the variable histologic growth patterns and the cytologic variability observed both in terms of the cell size, cell shape, and nuclear and cytoplasmic features. The most important feature that helps distinguish these tumors from other types of thymoma is their sheet-like growth pattern and paucity of lymphocytes, which imparts them with a distinctive “pink” appearance on scanning magnification, in contrast with types B1 and B2 which are more “blue” due to the abundance of lymphocytes. On higher magnification, the neoplastic cells are also decidedly different from those of conventional type B1 and B2 thymoma due to their increased nuclear to cytoplasmic ratio and increased nuclear chromatin pattern, features which underscore their more advanced stage of neoplastic transformation compared with the other conventional types of thymoma. Another distinguishing feature of these tumors is the highly cohesive nature of the tumor cells which are closely apposed and touching one another, unlike the singly scattered, discohesive appearance of the cells in B1 and B2 thymomas. The frequent transitions between areas of B1, B2 and B3 thymoma within the same tumor seen in many of our cases also highlights the close relationship that exists between the different histologic variants of thymoma, reinforcing the notion that they all correspond to different stages in the evolution of thymic epithelial neoplasia. Further support for the concept of the continuity that exists between the various histologic subtypes is the demonstration of transitions and combinations of atypical thymoma with thymic carcinoma in 16 cases (13%), highlighting the capability for progression of malignancy of these tumors, a feature that has been previously well-documented in the literature31.
The results of our immunohistochemical analysis in these cases showed that all tumors were consistently positive for p40/p63 and cytokeratin antibodies. None of the other markers tested showed any significant specificity or sensitivity for diagnosis. A very helpful finding was the demonstration of increased proliferative activity in atypical thymoma compared with the other types of thymoma as measured by MIB-1 immunostaining. On average, nuclear staining for this antibody was significantly increased (mean 11.6% nuclear positivity). Prior studies have shown that nuclear staining for types A, AB, B1 and B2 thymoma are in the order of 0–2%, making this a potentially helpful tool for distinguishing type B3 from the other types32. Given that there is some degree of variability in the intensity of staining for MIB-1 in atypical thymoma, we recommend caution with its use on small biopsy specimens in which the full features of the lesion may not be clearly appreciated, although identifying a higher (>5%) proliferation index should raise the possibility of an atypical component. Assessment of proliferative activity can also be of potential assistance for separating atypical thymomas from thymic carcinoma. Recent studies have demonstrated an average of 30% nuclear positivity in poorly differentiated non-keratinizing squamous cell thymic carcinoma, one of the most common types of thymic carcinoma33. Distinction of atypical thymoma from well-differentiated squamous cell carcinoma, however, can pose a more significant problem. This is particularly underscored by the fact that the squamoid variant of atypical thymoma often displays sharply delineated cell membranes imparting them with a pavement-like architecture (i.e., “squamoid” appearance), and that they can also display foci of abrupt keratinization. Distinction in such cases is made by paying attention to the sheet-like growth pattern of atypical thymoma and the abundance of perivascular spaces, features that should not be expected in squamous cell carcinoma which is generally characterized by irregular infiltrative islands of tumor cells separated by desmoplastic stroma displaying more striking cytologic atypia and mitotic activity. In small biopsy samples, however, the distinction may be rendered more difficult. Distinction between B2 and B3 thymoma is not as critical and can be facilitated by attention to the nuclear features (i.e., increased nuclear:cytoplasmic ratio and increased chromatin pattern in atypical thymoma) and by increase in proliferative activity as measured by MIB1 immunostaining.
Another significant finding in our study was the demonstration of positivity of the tumor cells for CD5 in 23 cases (25%), and for CD117 in 13 cases (14%). These markers have been generally associated with thymic carcinoma and their expression in tumors of the mediastinum has been widely accepted as an indication that one is likely dealing with a thymic carcinoma34,35,36. This can constitute a pitfall for diagnosis, particularly in small biopsy samples in which there may not be sufficient tissue to evaluate the overall architecture of the lesion; caution should be exercised in making a diagnosis of thymic carcinoma under such circumstances. Previous consensus guidelines proposed by ITMIG have also recommended that histologically typical B3 thymomas with expression of CD5 and CD117 should not be diagnosed as thymic carcinoma, underscoring the primacy of histologic examination over immunohistochemistry in this context14. It is also of interest that our statistical analysis did not reveal any differences in behavior and prognosis between the cases that were positive for CD5/CD117 and those that were negative, suggesting a spurious pattern of expression or cross-reactivity for these markers.
A more problematic scenario is encountered when making a distinction between the spindle cell type of B3 thymoma and the new category in the WHO classification of “atypical type-A thymoma”. In the last two WHO monographs, a new category of “atypical type A thymoma” was introduced that was defined as a tumor composed of spindle cells showing “some degree of atypia” such as hypercellularity, increased mitotic counts, and focal necrosis1. The current definition of B3 thymoma by the WHO is that of a tumor composed of mildly or moderately atypical polygonal tumor cells accompanied by small numbers of non-neoplastic immature T cells1. It is also mentioned in the latest WHO monograph that “whether a spindle cell variant of B3 thymoma exists is unclear, and distinction from atypical type A thymoma is difficult”, thereby implicitly acknowledging the existence of a problem area for these tumors. Criteria to separate this new category of type A thymoma from a potential B3 thymoma composed of spindle cells were not discussed. In a consensus conference based on a limited study of 7 cases of atypical type A thymoma, the two criteria that were agreed upon for diagnosis by the panel were increased mitotic activity (4 or more per 10 high power fields) and “true” coagulative necrosis13. Other diagnostic criteria such as hypercellularity, enlarged hyperchromatic nuclei, large nucleoli, and increased Ki-67 index were felt to be difficult to quantify or could not be agreed upon. In our experience, the latter criteria are sufficient to qualify a tumor for the designation of atypical thymoma of spindle cell type based on the simple fact that they display significantly increased cytologic atypia compared with conventional type A thymoma. The concept of a spindle cell type of thymic tumor equivalent to B3 thymoma predates the WHO proposal, and has been acknowledged as a distinct form of this tumor for many years29,37,38. We believe that “atypical type A thymoma” and B3 thymoma of spindle cell type are the same entity and we regard them as synonymous; therefore, there is currently no justification for the creation of a new category of “atypical type A thymoma”. Future molecular studies, however, may help further clarify this point of contention. Given the reported occurrence of transformation of type A thymoma into thymic carcinoma, and the progression to thymic carcinoma in atypical thymoma of spindle cell type seen in this study it is of importance to recognize this variant of atypical thymoma as distinct and separate from conventional type A thymoma and as a tumor harboring an increased potential for progression to a more advanced stage of malignancy, as demonstrated by the cases in our study.
The most important consideration for patient management is the biologic behavior of these tumors. Overall survival for these tumors has been variably reported to be from 50 to 70% at 10 years and 25 to 36% at 20 years15,17,39,40, with recurrences being observed in up to 44% of cases40. In our study, overall survival for our patients was 87% at 5 years, 67% at 10 years, and 23% at 20 years. Both Masaoka stage and status of resection margins after surgery were found to be significant prognostic factors for freedom from relapse and time to progression for these tumors. In a study of 188 patients from China addressing treatment response to WHO B3 thymoma, the 5 and 10-year survival rates were 91% and 73%, respectively; multivariate analysis showed that prognostic factors for overall survival included Masaoka stage and adjuvant radiation for patients with stage III and IV type B3 thymoma41. In a retrospective study of the ITMIG database, the overall survival rate for complete resection of B3 thymoma was 89% at 5 years and 82% at 10 years, and the recurrence rates were 23% at 5 years and 29% at 10 years22. The prognostic significance of the resection status, tumor size, and other parameters have not been determined for type B3 thymoma so far. Our study supports previous observations that the most significant prognostic parameters for these tumors are the status of resection and clinical staging at the time of diagnosis. Our study also shows an increase in the incidence of distant metastases, compared with an overall rate of 1–4% observed in a large meta-analysis of the published literature in conventional thymomas42. Another important observation in our study was that even for patients with recurrence and metastases, aggressive treatment with radiation and chemotherapy plus additional resection yielded favorable results. In 14 of our patients (cases 11, 31, 34, 35, 39, 42, 44, 49, 50, 51, 56, 94, 99 and 119), multiple recurrences and metastases occurred between 1 and 23 years after surgery and were successfully treated by surgery and combined modality therapy resulting in survivals ranging from 3 to 30 years from time of initial relapse to death (mean = 19.8 years). Previous studies have also demonstrated that aggressive treatment with combined chemotherapy, surgical resection and postoperative radiation therapy can produce long-term survival for patients with invasive thymomas43,44. This most likely reflects the slow growth of these tumors even after they have recurred or metastasized and offers a valid rationale for pursuing aggressive treatment for recurrences and metastases.
Given the importance of emerging molecular targeted therapies in the treatment and management of malignancies, we thought it would be important to evaluate the potential for targeted therapy in these tumors. Mutational status was analyzed by next generation sequencing in a subset of 47 cases using a panel of genes specifically addressing actionable genes for which targeted therapies currently exist. The results of our study showed no “actionable” molecular alterations, although the study was limited by the small size of the panel and the fact that it did not interrogate for possible fusions, gene amplifications, or other changes resulting from larger structural chromosomal abnormalities. CADD score, which is used to prioritize causal variants showed that RET p.A641S was the only variant identified (in only 3 patients) that is predicted as likely pathogenic. Overall, these tumors did not appear to have a significant burden of deleterious variants. Given the recent realization that some thymic neoplasms such as metaplastic thymoma are driven by recurrent gene fusions45, molecular testing with expanded sequencing panels might reveal additional alterations not detectable in this study.
In summary, we have delineated the spectrum of histopathologic, immunohistochemical and molecular genetic features in these tumors in a cohort of 120 patients and correlated them with their clinical behavior. Atypical thymoma is a rare type of primary thymic epithelial neoplasm displaying obvious increase in cytologic atypia compared with the other types of thymoma that can show a broad spectrum of morphologic appearances and has a clear potential for the progression of malignancy with the eventual development of thymic carcinoma. The most important parameters that correlate with clinical behavior are the clinical staging and the status of resection at the time of diagnosis. Complete surgical excision with clear margins offers the best chance for cure, while tumors in more advanced stages are prone to recurrence, metastases and progression of malignancy leading to death. Long-term follow-up with aggressive management of recurrences and metastases appears to represent a valid treatment approach for these tumors.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
WHO Classification of Tumors, 5th. Edition: Thoracic Tumors, WHO Classification of Tumors Editorial Board, IARC, Lyon, 2021.
Rosai J. Histological typing of tumors of the thymus, In World Health Organization International Histological Classification of Tumors, 2nd edn (eds Rosai J.) (Berlin, Springer-Verlag, 1999).
Koga, K. et al. A review of 79 thymomas: modification of staging system and reappraisal of conventional division into invasive and non-invasive thymoma. Pathol. Int. 44, 359–367 (1994).
R. Core team: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, 2021. https://www.R-project.org/.
Wang, K., Li, M. & Hakonarson, H. ANNOVAR: functional annotation of genetic variants from high-thoroughput sequencing data. Nucleic Acids Res. 38, e164 (2010).
Rentzsch, P., Witten, D., Cooper, G. M., Shendure, M. & Kircher, M. CADD: predicting the deleteriousness of variant throughout the human genome. Nucleic Acids Res. 47, D886–D894 (2019).
Suster, S. & Moran, C. A. Histologic classification of thymomas: The World Health Organization and beyond. Hematol. Oncol. Clin. NA 22, 381–392 (2008).
Lattes, R. Thymoma and other tumors of the thymus. Anal. 107 Cases Cancer 15, 1224–1260 (1962).
Bernatz, P. E., Harrison, E. G. & Clagget, O. T. Thymoma. A clinicopathologic study. J. Thorac. Cardiovasc. Surg. 42, 424–444 (1961).
Su, X. Y. et al. Immunohistochemical differentiation between type B3 thymomas and thymic squamous cell carcinomas. Int. J. Clin. Exp. Pathol. 8, 5354–5362 (2015).
Hayashi, A. et al. The evaluation of immunohistochemical markers and thymic cortical microenvironmental cells in distinguishing thymic carcinoma from type B3 thymoma or lung squamous cell carcinoma. J. Clin. Exp. Hematop. 53, 9–19 (2013).
Kojika, M. et al. Immunohistochemical differential diagnosis between thymic carcinoma and type B3 thymoma: diagnostic utility of hypoxic marker, GLUT-1, in thymic epithelial neoplasms. Mod. Pathol. 22, 1341–1350 (2009).
Kaira, K. et al. MUC1 expression in thymic epithelial tumors: MUC1 may be a useful marker as differential diagnosis between type B3 thymoma and thymic carcinoma. Virchows Arch. 458, 615–620 (2011).
Marx, A. et al. ITMIG consensus statement on the use of WHO histological classification of thymoma and thymic carcinoma: refined definitions, histological criteria and reporting. J. Thorac. Oncol. 9, 596–611 (2014).
Chen, G. et al. New WHO histologic classification predicts prognosis of thymic epithelial tumors: a clinicopathologic study of 200 thymoma cases from China. Cancer 95, 420–429 (2002).
Okumura, M. et al. The World Health Organization histologic classification system reflects the oncologic behavior of thymoma. A clinical study of 273 patients. Cancer 94, 624–632 (2002).
Okumura, M. et al. Oncological significance of WHO histological thymoma classification. A clinical study based on 286 patients. Jpn. J. Thorac. Cardiovasc. Surg. 50, 189–194 (2002).
Kondo, K. et al. WHO histologic classification is a prognostic indicator in thymoma. Ann. Thorac. Surg. 77, 1183–1188 (2004).
Sonobe, S. et al. Clinical usefulness of the WHO histological classification of thymoma. Ann. Thorac. Cardiovasc. Surg. 11, 367–373 (2005).
Wick, M. R. Prognostic factors for thymic epithelial neoplasms, with emphasis on tumor staging. Hematol. Oncol. Clin. NA 22, 527–542 (2008).
Marchevsky, A. M. et al. Evidence-based pathology and the pathologic evaluation of thymomas: The World Health Organization classification can be simplified into only 3 categories other than thymic carcinoma. Cancer 112, 2780–2788 (2008).
Weiss, C. A. et al. The impact of thymoma histotype on prognosis in a worldwide database. J. Thorac. Oncol. 10, 367–372 (2015).
Roden, A. C. et al. Modified Masaoka stage and size are independent prognostic predictors in thymoma and modified Masaoka stage is superior to histopathologic classifications. J. Thorac. Oncol. 10, 691–700 (2015).
Weissferdt, A. et al. Thymoma: a clinicopathological correlation of 1470 cases. Hum. Pathol. 73, 7–15 (2018).
Ruffini, E. et al. Tumors of the thymus: A cohort study of prognostic factors from the European Society of Thoracic Surgeons database. Eur. J. Cardiothorac. Surg. 46, 361–368 (2014).
Suster, S. & Moran, C. A. Problem areas and inconsistencies in the WHO classification of thymoma. Semin. Diagn. Pathol. 22, 188–197 (2005).
Suster, S. & Moran, C. A. Thymoma classification: current status and future trends. Am. J. Clin. Pathol. 125, 542–554 (2006).
Kirchner, T. et al. Well-differentiated thymic carcinoma. An organotypical low-grade carcinoma with relationship to cortical thymoma. Am. J. Surg. Pathol. 16, 1153–1169 (1992).
Suster, S. & Moran, C. A. Thymoma, atypical thymoma and thymic carcinoma. A novel conceptual approach to the classification of neoplasms of thymic epithelium. Am. J. Clin. Pathol. 111, 826–833 (1999).
Suster, S. & Moran, C. A. Primary thymic epithelial neoplasms. Spectrum of differentiation and histologic features. Semin. Diagn. Pathol. 16, 2–17 (1999).
Suster, S. & Moran, C. A. Primary thymic epithelial neoplasms with combined features of thymoma and thymic carcinoma. A clinicopathologic study of 22 cases. Am. J. Surg. Pathol. 20, 1469–1480 (1996).
Suster, D., Miller, J., Pihan, G., Mackinnon, A. C. & Suster, S. Expression Patterns of β-Catenin, E-Cadherin, PAX8, Bcl-2, EMA and MIB1 in Thymomas. Mod. Pathol. 34, 1831–1838 (2021).
Suster, D., Pihan, G., Mackinnon, A. C. & Suster, S. Poorly-differentiated nonkeratinizing squamous cell carcinoma of the thymus. Clinicopathologic and molecular genetic study of 25 cases. Am. J. Surg. Pathol. 42, 1224–1236 (2018).
Pan, C. C., Chen, P. C. H. & Chiang, H. KIT (CD117) is frequently overexpressed in thymic carcinomas but is absent in thymomas. J. Pathol. 202, 375–381 (2004).
Nakagawa, K. et al. Immunohistochemical KIT (CD117) expression in thymic epithelial tumors. Chest 128, 140–144 (2005).
Hosaka, N. et al. The role of CD5 expression in thymic carcinoma: possible mechanism for interaction with CD5+ lymphoid stroma (microenvironment). Histopathology 68, 450–455 (2016).
Nonaka, D. & Rosai, J. Is there a spectrum of cytologic atypia in type A thymomas analogous to that seen in type B thymoma? A pilot study of 13 cases. Am. J. Surg. Pathol. 36, 889–894 (2012).
Harris, N. L. & Muller-Hermelink, H. K. Thymoma classification: a siren’s song of simplicity. Am. J. Clin. Pathol. 112, 299–303 (1999).
Harnath, T. et al. Thymoma. A clinicopathological long-term study with emphasis on histology and adjuvant radiation dose. J. Thorac. Oncol. 7, 1867–1871 (2012).
Strobel, P. et al. Tumor recurrence and survival in patients treated for thymoma and thymic squamous cell carcinoma: a retrospective analysis. J. Clin. Oncol. 22, 1501–1509 (2004).
Gao, L. et al. Outcome of multimodality treatment for 188 cases of type B3 thymoma. J Thorac. Oncology 8, 1329–1334 (2013).
Suster S., Moran C. A. Chapter 17: The Mediastinum. In Modern Surgical Pathology, Vol.1, 2nd edn, (eds Weidner N., Cote R. J., Suster S., Weiss M. L). (Saunders (Elsevier), Philadelphia, 2009).
Modh, A. et al. Treatment modalities and outcomes in patients with advanced invasive thymoma or thymic carcinoma: A retrospective multicenter study. Am. J. Clin. Oncol. 39, 120–125 (2016).
Hishida, T. et al. Long-term outcome and prognostic factors of surgically treated thymic carcinoma: results of 306 cases from a Japanese Nationwide database study. Eur. J. Cardiothorac. Surg. 49, 835–841 (2016).
Vivero, M., Davineni, P., Nardi, V., Chank, J. K. C. & Sholl, L. M. Metaplastic thymoma: a distinctive thymic neoplasm characterized by YAP1-MAML2 gene fusions. Mod. Pathol. 33, 560–565 (2020).
Author information
Authors and Affiliations
Contributions
DS and SS performed the study concept and design and participated in the writing of the paper; ACM, GP and MB performed development of methodology and writing, review, and revision of the paper; MD performed statistical analysis, interpretation of the data, and writing of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval/consent to participate
The study was conducted under appropriate IRB approval for the participating institutions.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Suster, D.I., Craig Mackinnon, A., DiStasio, M. et al. Atypical thymomas with squamoid and spindle cell features: clinicopathologic, immunohistochemical and molecular genetic study of 120 cases with long-term follow-up. Mod Pathol 35, 875–894 (2022). https://doi.org/10.1038/s41379-022-01013-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-022-01013-x