Abstract
The transient receptor potential cation channel, subfamily M, member 1 (TRPM1/Melastatin-1/MLSN-1) expression has been shown to have prognostic utility in the evaluation of primary cutaneous melanoma. We analyzed a series of spindled and epithelioid cell nevi (Spitz) and primary cutaneous nodular melanomas to determine whether the expression of TRPM1 mRNA may be useful in distinguishing between Spitz nevi and nodular melanomas and to further examine the patterns of TRPM1 mRNA expression in cutaneous melanocytic proliferations. Formalin-fixed, paraffin-embedded tissues from 95 Spitz nevi and 33 nodular melanomas were analyzed for the expression of TRPM1 mRNA by in situ hybridization using 35S-labeled riboprobes. Ubiquitous melanocytic expression of TRPM1 mRNA was observed in 56 of 95 (59%) Spitz nevi and 4 of 33 (12%) nodular melanomas. Diffusely scattered loss of TRPM1 mRNA was identified in 38 of 95 (40%) Spitz nevi and 2 of 33 (6%) nodular melanomas. Regional loss of the TRPM1 mRNA expression by a significant subset of dermal tumor cells or a complete absence of TRPM1 expression by the dermal tumor was identified in 27 of 33 (82%) nodular melanomas, but only 1 of 95 (1%) Spitz nevi. These findings suggest that the pattern of TRPM1 mRNA expression may be helpful in the differentiation of Spitz nevi and nodular melanomas. Of the 16 patients who experienced metastasis, 15 (94%) had primary tumors that displayed reduced MLSN mRNA expression by all or a part of the dermal tumor.
Similar content being viewed by others
Main
Spindled and epithelioid cell nevi, also known as Spitz nevi, were originally reported as tumors arising predominantly in childhood. These tumors also occur in adults and in some cases may mimic melanoma histologically. Indeed, historically, spindled and epithelioid cell nevi have been described as histopathological stimulants of melanoma.1, 2, 3 Although many Spitz nevi can readily be distinguished from nodular melanomas, there remains a subset of tumors for which a definitive diagnosis of Spitz nevus versus that of melanoma is not rendered with ease.4, 5, 6 Of the benign histological mimics of melanoma, Spitz nevi are among the most important to be recognized because they often extend into the reticular dermis and have a tumor thickness that may provoke sentinel lymph node mapping if called melanoma. Although Spitz nevi are cured by local excision, the 5-year survival for patients with nodular melanoma involving the reticular dermis (level IV) is <50%.7 Given the markedly different treatment protocols and prognostic implications of the respective diagnoses, ancillary techniques that may assist in this distinction would be helpful.
Studies in human melanocytic tumors have indicated that TRPM1, also known as Melastatin (MLSN-1), mRNA expression has diagnostic and prognostic utility.8, 9, 10, 11 TRPM1 is a member of the transient receptor potential (TRP) channel family with as-yet determined function in melanocytic tumors. Channels in the TRP family allow for calcium entry into cells, producing intracellular responses linked to the phosphatidylinositol and protein kinase C signal transduction pathways.12 The promoter region of TRPM1 contains four consensus binding sites for MITF.13 TRPM1 is transcriptionally regulated by MITF, as is evidenced by the strong response of TRPM1 expression in vitro to MITF upregulation or downregulation.14 TRPM1 was identified by differential cDNA display in B16 murine melanoma cell lines of variable metastatic potential.13 In humans, all benign nevi reported to date have been shown to uniformly express TRPM1 mRNA, whereas primary melanomas show variable expression, and all melanoma metastases show at least regional loss of TRPM1 mRNA expression.8, 9
A study analyzing the histological patterns of TRPM1 mRNA expression in 64 cases of benign melanocytic nevi, primary cutaneous melanomas, and melanoma metastases found ubiquitous melanocytic expression of TRPM1 mRNA in all 14 cases of benign melanocytic nevi. On the other hand, 19 of 36 (53%) primary cutaneous melanomas and 11 of 11 (100%) melanoma metastases showed loss of TRPM1 mRNA expression in at least part of the tumor.9 A subsequent study of 150 patients with localized cutaneous melanoma (AJCC I and II) showed that decreased expression of TRPM1 mRNA in the primary cutaneous tumor correlated with an increased risk of developing metastasis. In multivariate analysis of TRPM1 mRNA expression and other clinical and pathological prognostic factors, TRPM1, tumor thickness, and mitotic activity were found to be independent and interactive predictors of disease-free survival.10
The goals of this study were to analyze the patterns of expression of TRPM1 mRNA in Spitz nevi and nodular melanomas and to determine whether specific patterns of expression of this novel melanocyte-specific gene correlate with diagnosis and patient outcome.
Materials and methods
Cases and Tissues
Patients were identified by review of the surgical pathology files of the James Homer Wright Pathology Laboratories at the Massachusetts General Hospital and by the records of the Massachusetts General Hospital Cancer Registry for the years 1988–1994. Formalin-fixed, paraffin-embedded tissue blocks were obtained from all available cases. These included 95 Spitz nevi and 33 nodular melanomas. Hematoxylin and eosin-stained sections were reviewed by three dermatopathologists (L.M.D., G.A.L., L.A.E.) for verification of diagnoses. Cases were evaluated microscopically for the following histological parameters: ulceration, mitotic activity, tumor diameter, tumor thickness, and Clark level. Clinical information including age, gender, and follow-up was obtained by chart review and discussion with the patients' physicians. This protocol was approved by the Institutional Review Board Subcommittee on Human Studies at the Massachusetts General Hospital (Boston, MA, USA (98-7327)).
In Situ Hybridization
In situ hybridization studies were carried out as reported previously.9 Formalin-fixed, paraffin-embedded tissue sections were cut at 4 μm thickness, deparaffinized, rehydrated, and postfixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 15 min. After washing with PBS, the tissue sections were digested with 2 μg/ml proteinase K at 37 °C for 15 min and incubated with 4% paraformaldehyde/PBS for 10 min. The tissue sections were then washed with PBS, incubated with 0.2 N HCl for 10 min and 0.25% acetic anhydride/1 mol/l triethanolamine for 10 min, and dehydrated with graded ethanols. Hybridizations were carried out with single-stranded 35S-radiolabeled (5 × 107 c.p.m./ml) cRNA probes encoding a 1.9-kb segment of the coding region of the human cDNA (accession no. AF071787)8 or a 1-kb segment of the coding region of the human H4 histone gene in the presence of 50% formamide, 10% dextran sulfate, 1 × Denhardt's solution, 600 mmol/l NaCl, 10 mmol/l DTT, 0.25% sodium dodecyl sulfate, and 100 μg/ml tRNA for 18 h at 55 °C. After hybridization, the slides were washed with 5 × standard saline citrate (SSC) at 55 °C for 30 min, 50% formamide/2 × SSC at 55 °C for 30 min, 10 mmol/l Tris-HCl (pH 7.6)/500 mmol/l NaCl/1 mmol/l EDTA (TNE buffer) at 37 °C for 10 min, incubated once in 2 × SSC at 50 °C for 30 min, twice in 0.2 × SSC at 50 °C for 30 min, and dehydrated with graded ethanols. Localization of mRNA transcripts was detected by dipping the slides in Kodak NTB2 photoemulsion (Eastman Kodak, Rochester, NY, USA) and exposing them for 10–14 days at 4 °C.15 Before dehydration and coverslipping of the slides, the sections were counterstained using Myer's hematoxylin and alcoholic eosin Y. H4 histone probes were used as positive controls, and sense probes were used as negative controls.
Scoring TRPM1 Assay
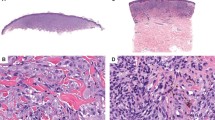
Loss of TRPM1 expression was determined by identifying the loss of TRPM1 mRNA expression in tumor cells as compared with the background levels of photoemulsion grains. Examples of scoring are seen in Figures 1 and 2. All melanocytic proliferations showed TRPM1 mRNA expression throughout the intraepidermal component. Slides were scored as having ‘no loss’ if TRPM1 expression was equally detectable at all levels of the intradermal extension. ‘Scattered loss’ was defined as reduced TRPM1 mRNA signal scattered randomly throughout the tumor (another term may be ‘diffuse loss’; we prefer the term ‘scattered’ to indicate that only few cells randomly distributed show downregulation of mRNA). ‘Regional loss’ was defined as focal areas or well-defined regions of the tumor exhibiting only background signal of TRPM1 mRNA. Although the term ‘focal loss’ was also considered for this pattern, the focus of reduced TRPM1 mRNA expression may actually be quite large, for example, more than a focus of a few cells. ‘Complete loss’ was defined as uniform loss of TRPM1 mRNA expression by all dermal tumor cells (Figure 1). Benign intraepidermal melanocytes elsewhere in the sections were used as internal positive controls for sample RNA integrity because they normally express high levels of MLSN mRNA. In addition, tumors displaying complete loss of TRPM1 mRNA expression were examined for H4 histone expression to confirm mRNA preservation. All cases displayed TRPM1 mRNA expression in intraepidermal melanocytes. TRPM1 mRNA expression for each tumor was scored independently by three dermatopathologists (L.A.E., G.A.L., L.M.D.) in the absence of patient outcome information. Discordant scoring results between readers were resolved by joint review.
Schematic representation of scoring for TRPM1 mRNA expression in cutaneous melanocytic tumors. All melanocytic proliferations show TRPM1 mRNA expression throughout the intraepidermal component. No loss was defined as TRPM1 mRNA expression equally detectable at all levels of the intradermal extension. Scattered loss (diffuse loss) was defined as reduced TRPM1 mRNA signal scattered randomly throughout the tumor. Regional loss (focal loss) was defined as focal areas or well-defined regions of the tumor exhibiting only background signal of TRPM1 mRNA. Complete loss was defined as uniform loss of expression of TRPM1 mRNA by all dermal tumor cells.
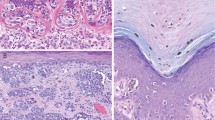
Examples of TRPM1 mRNA expression patterns. In situ hybridization using 35S-labeled riboprobe; white grains correlate with positive TRPM1 mRNA expression in dark field images. All melanocytic proliferations showed TRPM1 mRNA expression throughout the intraepidermal component. No mRNA loss: (a) H&E, (b) TRPM1 dark field, Spitz nevus. Scattered loss: (c) H&E, (d) TRPM1 dark field, Spitz nevus. Regional loss: (e) H&E, (f) TRPM1 dark field, nodular melanoma, reduced TRPM1 expression is observed in the dermal tumor cells in the lower half of the field. The irregular side-to-side color shading in the bright field images is a result of irregular uptake of H&E by the coating of NTB2 photographic emulsion.
Statistical Analysis
Statistical analysis was carried out using the SAS statistical package version 6.12 (SAS Institute Inc., Cary, NC, USA). The association of TRPM1 mRNA status with other covariates was tested with the χ2 test for categorical data and the Wilcoxon test.
Results
The tumors in this study showed characteristic histological features. The Spitz nevi were symmetrical circumscribed melanocytic proliferations composed of large nests of melanocytes separated by cleft-like spaces from the adjacent and overlying hyperplastic epidermis. Cytologically, the Spitz nevi showed an admixture of epithelioid and spindled melanocytes with large nuclei, evenly distributed chromatin, smooth nuclear margins, prominent central nucleoli, and abundant eosinophilic cytoplasm. Nodular melanomas were composed of proliferations of cytologically atypical melanocytes with increased nuclear to cytoplasmic ratios, clumped chromatin, and irregular nuclear outlines. The nodular melanomas were thicker on average than Spitz nevi (median Breslow thickness: 2.7 mm (0.5–14.7 mm) vs. 0.5 mm (0.1–3.2 mm), respectively, (P=0.027)). Ulceration was identified in 1 of 95 Spitz nevi and in 14 of the 33 nodular melanomas. Mitotic activity was identified in 22 of 95 of the Spitz nevi, but no Spitz nevus showed greater than 1 mitotic figure per millimeter squared. Of the 33 nodular melanomas, 15 showed more than one mitoses per square millimeter. Mitoses were identified in the deep aspect of the nodular melanomas, but not in the deep aspect of the Spitz nevi.
The clinical features of the patients with Spitz nevi and those with nodular melanomas are summarized in Table 1. The median age of patients with Spitz nevi was less than those with nodular melanomas (24 vs. 60 years; P=0.012). The site distribution of Spitz nevi and melanoma was similar with approximately half of cases occurring on the extremity (48 vs. 52%), one-third on the trunk (32 vs. 30%), one-fifth on the head and neck (17 vs. 18%), and the remainder at other sites. Clinical follow-up data beyond 9 months of diagnosis was available for 58 of the patients with Spitz nevi; with a mean follow-up duration exceeding 10 years (range 9 months to 20 years). No patient with a Spitz nevus developed metastatic disease. The mean duration of follow-up for the patients with nodular melanomas was 7.6 years (range 1–17). Of the 33 patients with nodular malignant melanoma, 17 did not experience metastasis, 5 patients were alive but had suffered metastases, and 11 patients died from complications related to metastatic melanoma. Of the patients who died, the mean length of time from diagnosis to death was 2.6 years (range 1–7).
TRPM1 mRNA was detected in bright-field microscopy as black grains overlying the cells and in dark field as bright white spots (Figure 2). Normal epidermal melanocytes expressed TRPM1 mRNA. In cases of melanoma with contiguous benign nevi, the benign nevomelanocytic component showed uniform TRPM1 mRNA expression. TRPM1 mRNA was detected in the intraepidermal component of the melanoma in all of the tumors tested. In contrast, variable expression of TRPM1 mRNA was observed in the dermal component of many of the Spitz nevi and the nodular melanomas. The results of the in situ hybridization assays are summarized in Table 2 and shown graphically in Figure 3.
Ubiquitous melanocytic expression of TRPM1 mRNA was observed in 56 of 95 (59%) Spitz nevi and 4 of 33 (12%) nodular melanomas; this uniform expression was termed ‘no loss.’ Scattered loss of TRPM1 mRNA was identified in 38 of 95 (40%) Spitz nevi and 2 of 33 (6%) nodular melanomas. Regional loss (focal loss) of TRPM1 mRNA was identified in 22 of 33 (67%) nodular melanomas; 1 Spitz nevus of 95 cases (1%) showed regional TRPM1 mRNA loss. Complete loss of detectable TRPM1 mRNA was manifested by the dermal component of 5 (15%) of the nodular melanomas but none of the Spitz nevi.
Expression of TRPM1 mRNA was evaluated with disease progression in patients with nodular melanoma. Four cases of NMM showed ubiquitous melanocytic TRPM1 mRNA expression; none died of disease. Three of four were without recurrence or metastasis; one developed metastatic disease 8 years after diagnosis and remained alive 7 years later. Two cases of NMM showed scattered loss of TRPM1 mRNA; in one case the patient was alive with no disease at last follow-up and in the other case the patient died with melanoma. Overall, 22 cases displayed regional loss of TRPM1 mRNA expression; 12 of these 22 experienced metastasis. Two of five patients with complete TRPM1 mRNA loss died with metastatic melanoma (Table 3).
Although the pattern association of TRPM1 mRNA expression with diagnosis of Spitz nevus or nodular melanoma is strong, we also sought to determine the impact of TRPM1 expression on the ability to improve upon existing diagnostic models.4 In multivariate analysis, all traditional variables were significantly associated with the diagnosis of Spitz nevus or melanoma (patient age, tumor thickness, ulceration, and mitotic activity) with the exception of tumor diameter. The addition of TRPM1 pattern of expression in the logistic regression model resulted in no significant improvement in the model. Reduced TRPM1 mRNA expression was observed to correlate with increased patient age in Spitz nevi (P=0.06) and with mitoses (P=0.07) and ulceration (P=0.01) in nodular melanoma.
Discussion
There were striking differences in TRPM1 mRNA expression between Spitz nevi and nodular melanomas. Most Spitz nevi showed no loss of TRPM1 mRNA (56 of 95 (69%) tumors), whereas this was infrequent in nodular melanoma (4 of 33 (12%) tumors). Regional (focal) or complete loss of TRPM1 was observed rarely in Spitz nevi (1 of 95 (1%)), but was common in nodular melanoma (27 of 33 (82%)). These findings are consistent with prior reports that TRPM1 expression is inversely correlated with biological aggressiveness in melanocytic lesions. Benign nevi are reported to ubiquitously express TRPM1 mRNA, whereas primary melanomas show variable TRPM1 expression.8
Interestingly, of the four patients with nodular melanomas showing no loss of TRPM1 mRNA expression, only one developed metastasis; this patient remains alive 15 years after primary melanoma diagnosis. In a previous study of patients with AJCC stage I melanoma, patients whose tumors did not show loss of TRPM1 mRNA were found to have an 8-year disease-free survival rate of 100%, whereas patients with primary tumors that showed TRPM1 loss had a disease-free survival of 77%.10 Patients with stage II disease whose tumors ubiquitously expressed TRPM1 mRNA had an 8-year disease-free survival of 90%, whereas patients with tumors showing TRPM1 loss had a disease-free survival rate of 51%. This study indicated that TRPM1 mRNA expression has prognostic significance in nodular melanomas.
Of the four patterns of TRPM1 expression, perhaps the least understood is that of ‘scattered loss.’ Scattered loss was manifested by an overall random reduction of mRNA expression by scattered dermal tumor cells. This pattern of expression is reminiscent of the gradient of expression observed for other markers in benign nevi, in that there was no clear focus of marker loss. Indeed, one possible explanation is that mRNA levels are reduced in randomly scattered cells to a level below the detection of this assay, possibly a low level of undetectable mRNA remains. In the future, the availability of a reliable colorimetric in situ hybridization assay for TRPM1 may help in further evaluation of expression patterns in melanocytic tumors. Although there is a published study using a colorimetric technique, this assay was not deemed by those authors to be a significant improvement over the radioactive assay.10 Unfortunately, no immunohistochemical reagent exists to detect TRPM1 protein in tissue sections. Correlation of mRNA and protein levels would certainly help to further understand the patterns of TRPM1 expression in melanocytic tumors.
Regional loss (focal loss) or complete loss of TRPM1 mRNA was identified in 27 of 33 (82%) nodular melanomas, but only 1 of 95 (1%) Spitz nevi. Different histological patterns of TRPM1 mRNA expression have been reported to assist in distinguishing benign from malignant melanocytic neoplasms.9 Acquired melanocytic nevi always show ubiquitous melanocytic expression of TRPM1 mRNA in contrast to primary and metastatic melanomas, which often display regional or complete loss of expression. Loss of TRPM1 mRNA in a portion of the dermal component of an invasive melanoma has been reported to occur more frequently in primary cutaneous melanomas (19 of 36 cases, 53%) and in melanoma metastases (11 of 11 cases, 100%), but never in benign melanocytic proliferations.9 These findings correlate well with the observation of regional loss of TRPM1 mRNA expression in the majority of nodular melanomas in this study, but in only 1 of 95 Spitz nevi (Figure 4).
Although the histological pattern of loss of TRPM1 mRNA expression may be especially helpful in assisting in the differentiation of Spitz nevi from nodular melanomas, it is important to note that other factors including patient age, tumor thickness, mitotic activity, and ulceration are important diagnostic factors in this differential diagnosis.4 The addition of TRPM1 expression to a logistic regression model including these traditional factors did not improve the diagnostic model. It remains unclear whether TRPM1 expression would be useful in the analysis of atypical Spitz tumors and their differentiation from nodular melanoma.
The observation of variable TRPM1 expression in the dermal component of nodular melanomas is an example of intratumoral heterogeneity. Intratumoral heterogeneity is evident in the vertical growth phase of primary melanomas with respect to expression and distribution of a number of biomarkers as revealed by immunohistochemical and in situ hybridization analysis. For example, CEACAM protein is expressed at the advancing front of tumors,16 whereas reduced TRPM1 mRNA is displayed within subsets of tumor cells. The melanoma tumor suppressor, originally named melanoma differentiation associated gene-7 (mda-7)17, 18 and more recently recognized to be a melanocyte-derived cytokine and named IL-24,19 is observed in all benign melanocytic proliferations, and variably expressed in primary cutaneous melanomas with reduced expression in some subsets of vertical growth-phase tumor cells.9, 20 Benign nevi also display intratumoral heterogeneity; however, the pattern of expression is usually a gradient from superficial to deep dermal lesion. This gradient of expression has also been observed in primary melanomas that are cured by local excision.21
In the comparison of Spitz nevi with nodular melanomas, investigators have reported the expression of a number of markers including MART-1,22 CD44,23 NM23,24 and CD40.25 These observers report variable expression in nodular melanomas as compared with Spitz nevi. More specifically, although the expression of these markers was relatively uniform in Spitz nevi, loss of expression of MART-1 and CD44 and increased expression of CD40 were reported in some primary melanomas. Overall, the awareness of intratumoral heterogeneity in melanocytic tumors may guide future investigations of melanoma biomarkers.
Biomarker expression within tumors reflects a dynamic nature of tumors. In contrast to the historical view that tumors represent clonal proliferations of abnormal cells, all of which contain a common phenotype and/or genotype, malignant tumors are now understood to be composed of a heterogeneous mix of cells that display cytological distinctions and immunophenotypic and genotypic heterogeneity. Metastatic melanoma samples also display heterogeneity within a tumor sample and between tumor samples.26 Intratumoral heterogeneity may confound the interpretation of molecular profiling, given that any tumor may contain subsets of cells with distinct molecular profiles. In some cases, the tumor population bearing the metastasis-associated profile may represent a minor subset of the tumor as a whole. Identifying the molecular and immunophenotypic features of tumor subsets that are responsible for metastasis may be important in the identification of therapeutic targets for patients with melanoma.
In summary, intratumoral heterogeneity of TRPM1 mRNA expression was observed in both Spitz nevi and nodular melanomas. The pattern of TRPM1 mRNA expression may be a useful diagnostic marker because it is expressed in different histological patterns in Spitz nevi and nodular melanomas. Although observed in more than 80% of nodular melanomas, a pattern of regional (focal) or complete TRPM1 mRNA loss was rarely seen in Spitz nevi (1%). In addition, regional or complete loss of TRPM1 mRNA in primary cutaneous melanoma correlates with an increased risk of developing metastatic melanoma.
Accession codes
References
Darier J, Civatte A . Naevus ou naevo-carcinoma chez un nourisson. Bull Soc Fr Derm Syph 1910;21:61–63.
Pack G, Persik S, Scharfnagel I . Treatment of malignant melanoma: report of 862 cases. Calif Med 1947;66:283–287.
Spitz S . Melanomas of childhood. Am J Pathol 1948;24:591–609.
Spatz A, Calonje E, Handfield-Jones S, et al. Spitz tumors in children: a grading system for risk stratification. Arch Dermatol 1999;135:282–285.
Allen AC, Spitz S . Malignant melanoma: a clinicopathological analysis of the criteria for diagnosis and prognosis. Cancer 1953;6:1–45.
Barnhill RL, Argenyi ZB, From L, et al. Atypical spitz nevi/tumors: lack of consensus for diagnosis, discrimination from melanoma, and prediction of outcome. Hum Pathol 1999;30:513–520.
Balch CM, Soong S-J, Gershenwald JE, et al. Prognostic factors analysis of 17 600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol 2001;19:3622–3634.
Duncan LM, Deeds J, Hunter J, et al. Down-regulation of the novel gene melastatin correlates with potential for melanoma metastasis. Cancer Res 1998;58:1515–1520.
Deeds J, Cronin F, Duncan LM . Patterns of melastatin mRNA expression in melanocytic tumors. Hum Pathol 2000;31:1346–1356.
Duncan LM, Deeds J, Cronin FE, et al. Melastatin expression and prognosis in cutaneous malignant melanoma. J Clin Oncol 2001;19:568–576.
Hammock L, Cohen C, Carlson G, et al. Chromogenic in situ hybridization analysis of melastatin mRNA expression in melanomas from American Joint Committee on Cancer stage I and II patients with recurrent melanoma. J Cutan Pathol 2006;33:599–607.
Clapham DE, Runnels LW, Strubing C . The TRP ion channel family. Nat Rev Neurosci 2001;2:387–396.
Hunter JJ, Shao J, Smutko JS, et al. Chromosomal localization and genomic characterization of the mouse melastatin gene (MLSN-1). Genomics 1998;54:116–123.
Miller AJ, Du J, Rowan S, et al. Transcriptional regulation of the melanoma prognostic marker melastatin (TRPM1) by MITF in melanocytes and melanoma. Cancer Res 2004;64:509–516.
Lee K, Lanske B, Karaplis AC, et al. Parathyroid hormone-related peptide delays terminal differentiation of chondrocytes during endochondral bone development. Endocrinology 1996;137:5109–5118.
Thies A, Moll I, Berger J, et al. CEACAM1 expression in cutaneous malignant melanoma predicts the development of metastatic disease. J Clin Oncol 2002;20:2530–2536.
Madireddi MT, Su ZZ, Young CS, et al. Mda-7, a novel melanoma differentiation associated gene with promise for cancer gene therapy. Adv Exp Med Biol 2000;465:239–261.
Madireddi MT, Dent P, Fisher PB . Regulation of mda-7 gene expression during human melanoma differentiation. Oncogene 2000;19:1362–1368.
Caudell EG, Mumm JB, Poindexter N, et al. The protein product of the tumor suppressor gene, melanoma differentiation-associated gene 7, exhibits immunostimulatory activity and is designated IL-24. J Immunol 2002;168:6041–6046.
Ellerhorst JA, Prieto VG, Ekmekcioglu S, et al. Loss of MDA-7 expression with progression of melanoma. J Clin Oncol 2002;20:1069–1074.
Bouffard D, Duncan LM, Howard CA, et al. Actin-binding protein expression in benign and malignant melanocytic proliferations. Hum Pathol 1994;25:709–714.
Bergman R, Azzam H, Sprecher E, et al. A comparative immunohistochemical study of MART-1 expression in Spitz nevi, ordinary melanocytic nevi, and malignant melanomas. J Am Acad Dermatol 2000;42:496–500.
Ichikawa T, Masumoto J, Kaneko M, et al. Moesin and CD44 expression in cutaneous melanocytic tumours. Br J Dermatol 1998;138:763–768.
Lee CS, Pirdas A, Lee MW . Immunohistochemical demonstration of the nm23-H1 gene product in human malignant melanoma and Spitz nevi. Pathol 1996;28:220–224.
van den Oord JJ, Maes A, Stas M, et al. CD40 is a prognostic marker in primary cutaneous malignant melanoma. Am J Pathol 1996;149:1953–1961.
Zubovits J, Buzney E, Yu L, et al. HMB-45, S-100, NK1/C3, and MART-1 in metastatic melanoma. Hum Pathol 2004;35:217–223.
Acknowledgements
The authors thank Jeanette J. McCarthy, Frank E. Cronin, and Jim Deeds (formerly at Millennium Pharmaceuticals Inc., Cambridge, MA), and Tricia Della Pella (MGH Department of Pathology) for their contributions to this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Erickson, L., Letts, G., Shah, S. et al. TRPM1 (Melastatin-1/MLSN1) mRNA expression in Spitz nevi and nodular melanomas. Mod Pathol 22, 969–976 (2009). https://doi.org/10.1038/modpathol.2009.56
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2009.56
Keywords
This article is cited by
-
Role of TRP ion channels in cancer and tumorigenesis
Seminars in Immunopathology (2016)
-
Autoantibody against transient receptor potential M1 cation channels of retinal ON bipolar cells in paraneoplastic vitelliform retinopathy
BMC Ophthalmology (2012)
-
TRPA1 is functionally expressed in melanoma cells but is not critical for impaired proliferation caused by allyl isothiocyanate or cinnamaldehyde
Naunyn-Schmiedeberg's Archives of Pharmacology (2012)