Abstract
Topoisomerase IIα and minichromosome maintenance protein 2 are proteins associated with aberrant S-phase induction. The current study evaluated the performance of these biomarkers (ProEx™ C; TriPath Oncology, Burlington, NC) compared with p16INK4A and MiB-1 in distinguishing high-grade squamous intraepithelial lesions (HSILs) from HSIL mimics. We collected archival cervical biopsy, cone, and curettage specimens from 96 cases in which the differential diagnosis of HSIL vs reactive epithelial changes was considered. Hematoxylin- and eosin-stained slides were reviewed independently by three pathologists and scored for the presence or absence of SIL. Immunostains for ProEx C, p16, and MiB-1 were available for 95, 96, and 59 samples, respectively, and classified blinded to histological interpretation. Strong nuclear and cytoplasmic staining for p16 and staining for MiB-1 and ProEx C that extended beyond the lower one-third of the epithelium were scored as positive. χ2-tests and receiver operating characteristic analysis were conducted to statistically compare biomarker immunostaining performance against majority histological interpretation of SIL. Agreement between pathologists was also assessed by the κ-statistic. Inter-observer agreement ranged from fair to moderate (κ=0.37–0.57). All three biomarkers correlated strongly with the majority diagnosis of SIL (P<0.001). Positive staining for ProEx C, p16, and MiB-1 was observed in 87% (N=52/60), 84% (N=51/61), and 94% (34/36), respectively, of SIL and negative in 71% (N=25/35), 63% (N=22/35), and 52% (N=12/23), respectively, of majority diagnoses of NoSIL. The combination of p16/ProEx C predicted more SIL (92%, N=33/36) and NoSIL (61%, N=14/23) than p16 plus MiB-1 (94%, N=34/36 and 43%, N=10/23), although this difference was not statistically significant. ProEx C appears to provide an equivalent level of sensitivity and a higher level of specificity for HSIL alone or in conjunction with p16. Its principal value may be in providing a lower false positive rate for NoSIL relative to MiB-1.
Similar content being viewed by others
Main
It is well established that most cervical cancers develop from precursor lesions.1 High-grade squamous intraepithelial lesions (HSILs) are at greatest risk for a malignant outcome due to their stronger association with cancer-associated human papillomaviruses (HPVs), underscoring the importance of accurate histological classification.2, 3, 4
Histopathology is the method of choice for confirming the diagnosis of a squamous intraepithelial lesion (SIL) that is usually first detected by cytological screening. Diagnosis variability has been documented among observers and depends, in part, on the grade of the abnormality.4 Reactive/reparative epithelial changes, immature squamous metaplasia, and atrophy are well-recognized mimics of HSIL and frequently cause problems in histological interpretation.5
In HPV-infected squamous cells, E6 and E7 viral oncoproteins bind host regulatory proteins, leading to degradation of p53 protein and inactivation of the retinoblastoma gene protein Rb, both tumor suppressor gene products. These interactions ultimately lead to a deregulation of the cell cycle that is manifested by an abnormal expression of cell cycle-associated proteins. A number of cell cycle proteins have been used as biomarkers that can help to differentiate between SIL and mimics of SIL. Surrogate markers of SIL include Ki67, an antigen expressed in the nuclei of proliferating cells and detected with the MiB-1 antibody. The p16 protein is a cyclin-dependent kinase inhibitor that downregulates progression through the G1–S transition checkpoint of the cell cycle.6 Two newer biomarkers include the minichromosome maintenance protein 2 (MCM2) and DNA topoisomerase IIα (TPO2A). MCM2 functions during DNA replication by loading the pre-replication complex onto DNA and unwinding the DNA through helicase activity to permit DNA synthesis. DNA TOP2A is responsible for the enzymatic unlinking of DNA strands during replication. These two proteins play a significant role in the regulation of DNA replication during S-phase and are overexpressed when S-phase cell cycle induction is aberrant. MCM2 and TOP2A were both recently identified by gene expression studies,7 and ProEx C is a cocktail of two monoclonal antibodies that targets the expression of these two proteins.
In the present study, we compared the performance of ProEx C as a complementary surrogate marker to p16 and Ki67 in a set of tissue sections containing histological changes that are included in the differential diagnosis of HSIL.
Materials and methods
Case Selection
Diagnostically challenging cases were intentionally overrepresented in this study by selecting consecutive archival cervical specimens if they had a diagnosis of HSIL or a squamous reactive/atrophic process and there was documentation that immunohistochemistry had been performed at the time of histological diagnosis for p16 and/or Ki67. Between January 2005 and September 2007, 98 archival cervical specimens met these criteria. The cases consisted of 58 cervical biopsies, 20 endocervical curettages, 20 cone products, and 1 endocervical polyp. Eight cases had multiple sections from different locations in the cervix from the same patient. If hematoxylin and eosin (H&E) slides were missing from the file, sections were re-cut from the paraffin block. All H&E-stained slides were reviewed independently by three pathologists (APP, CPC, and EC) blinded to the original and the others' diagnoses and classified as SIL or not SIL (NoSIL) (ie, normal, reactive, and/or atrophic epithelium). A majority diagnosis for each case was based on the agreement of at least two of the three reviewers.
Immunohistochemistry
Four-micrometer sections of formalin-fixed, paraffin-embedded tissue were cut and placed on glass slides. Tissue sections were deparaffinized and rehydrated through graded alcohols. Endogenous peroxidase activity was blocked by incubation in 3% H2O2. Antigen retrieval was carried out with 10 mM citrate buffer (pH 6.0) and pressure cooker heat induction8 (122–125°C) for 30 s at 15–24 psi.
The p16 antigen was identified using the purified mouse anti-human p161NK4 monoclonal antibody (clone G175-405; Pharmingen International catalog no. 13251A, San Diego, CA, USA), concentration 1:400. Ki67 was identified using the mouse monoclonal antibody MIB-1 (Dakocytomation, CA, USA) at a concentration of 1:200. Application of the primary antibodies was followed by incubation with Dakocytomation anti-mouse IgG as a secondary antibody with 3,3′-diaminobenzidine (DAB) as a chromagen and Mayer hematoxylin counterstaining.
Staining for ProEx C was performed on 98 samples. The ProEx C antigen was identified using the prediluted (unknown concentration) mouse anti-human ProEx C monoclonal antibody (Tripath Imaging Inc, Burlington, NC, USA). Application of the primary antibody was followed by incubation with Dakocytomation anti-mouse IgG as the secondary antibody with DAB as the chromagen and Mayer hematoxylin counterstaining. Sections containing normal epithelia were included in each run to assess the background. Positive controls (slides containing HSIL) for all the three antibodies were included in each staining run.
Interpretation of immunostainings
One of the authors (APP) independently reviewed the immunostains. They were reported in a semiquantitative fashion and classified based on two main criteria for all antigens: distribution and intensity. The staining intensity was graded as weak (light brown) or strong (dark brown) for all antigens. For p16, a case was scored as weak when the staining was weak to moderate in both nuclei and cytoplasm, and as strong when it was strong in both nuclei and cytoplasm. For Ki67 and ProEx C, the predominant (more or less than 50%) type of stained nuclei (weak or strong) was considered. Distribution was adapted from Klaes et al9 and graded as <1, 1–5, 5–25, and >25% of positive cells. Distribution was further classified into horizontal for p16 and vertical for Ki67 and ProEx C. In horizontal distribution,6 the staining was scored as diffuse if it was seen continuously in the horizontal plane, either partial or full thickness. If it was interrupted in this plane, so that less than 80% of the epithelium stained positive, the staining was scored as patchy or focal. The grade on vertical plane was adapted from Shi J et al10 as follows: 1+, basal 1–2 layers; 2+, lower one-third; 3+, lower two-thirds; and 4+, more than the lower two-thirds to full thickness of the epithelial lesion.
Final scoring was assigned as negative in the following situations: (1) 0–5% of positive cells present for all stains; (2) weekly diffuse ‘blush’ staining for p16; (3) absence of nuclear staining independently of the grade of cytoplasmic staining for p16. Cases with >5% positive cells were scored as positive for p16, but negative for Ki65 and ProEx C if those cells where confined to the lower one-third of the epithelium (1+ or 2+). All other possibilities were scored as positive.
Statistical Analysis
Univariate χ2-tests and multivariable logistic regression were used to assess associations between biomarker immunostaining results and consensus diagnosis of SIL. P<0.05 was considered statistically significant. κ-Statistic was used to assess the degree of inter-observer agreement higher than that expected by chance on the H&E histological diagnosis. κ-=0 and 1 reflected no agreement and perfect agreement, respectively. Sensitivity and specificity values were calculated for each test and for combinations of both tests. Test accuracy for each immunostain as well as for each combination was assessed by receiver operating characteristic (ROC) curve analysis against final diagnosis established by the consensus panel of histological analysis. ROC curves reflecting combined test sensitivity and 1-specificity were plotted. The area under the ROC curves was compared for each indicator.11 ROC areas of 0.5 or less indicate diagnostic test markers that cannot distinguish cases of SIL from absence of lesion.
Results
Agreement on the diagnosis based on H&E alone ranged from fair to moderate (κ=0.37–0.59). All three biomarkers correlated positively with the majority diagnosis of SIL (P<0.001). Table 1 shows the relative sensitivity and specificity for each immunostain test and paired combination. Positive staining for ProEx C, p16, and MiB-1 was observed in 87, 84, and 94% of SIL cases, respectively. A significant predictive association between positive immunostaining and detection of SIL was observed for each antibody, independent of the patient age, specimen type (cervical biopsy, cone, or curettage), and number of re-cuts performed (data not shown). Furthermore, unlike MiB-1, which was highly correlated with both p16 and ProEx C, ProEx C immunostaining remained independently associated with diagnosis of SIL (ie, even after concurrent adjustment for p16 positivity). In contrast to the results for test sensitivity, negative immunohistochemical results were seen for ProEx C, p16, and MiB-1 in 71, 63, and 52% of majority diagnoses of NoSIL, respectively. Test accuracy, determined by the area under the ROC curve was highest for ProEx C (0.79) (Figure 1) and the combination of p16 and ProEx C (0.76) (Figure 2). The combination of p16 and ProEx C predicted more NoSIL (61%) than p16 and MiB-1 (43%) (Table 1). Gains in sensitivity observed by combining antibody tests were at the expense of poorer specificity, particularly when involving immunostaining results for MiB-1.
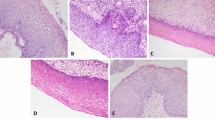
Examples of cases are illustrated in Figures 3 and 4. An HSIL with an immature metaplastic phenotype and positivity for all three tested antigens is shown in Figure 3a. Some disagreements among the reviewers in grading a SIL were seen in a small subset (less than 5%) of the lesions. For this reason, positive cases were designated as SIL instead of HSIL. Figure 3b shows a low-grade squamous intraepithelial lesion (LSIL) where both morphological diagnosis and grading are difficult. In this case, all immunoassays tested positive, but ProEx C showed the most intense signal. Figure 3c shows an HSIL with a higher degree of differentiation than in Figure 3a, but still with an immature phenotype. In this case, ProEx C stained a significantly higher number of cells than MiB-1. Three cases of immature metaplastic epithelium with variable degrees of atypia are shown in Figure 4. Figure 4a shows a squamous reparative epithelium diagnosed by the three reviewers as NoSIL. MiB-1 was positive due to the presence of nuclear signal beyond the lower one-third of the epithelium, whereas p16 and ProEx C were negative in this case. A case diagnosed as SIL by 2/3 reviewers is shown in Figure 4b. Although P16 was negative, MiB-1 and ProEx C were positive in this case. Finally, an immature metaplastic squamous epithelium with mild atypia diagnosed as NoSIL by the three reviewers is shown in Figure 4c. ProEx C was negative and P16 positive in this case.
Squamous intraepithelial lesions (three cases). (a1) A case diagnosed by 3/3 reviewers as HSIL and confirmed by the biomarker panel. All stain signals occur in more than 25% lesional cells. (a2) The p16 signal is strong and diffusely distributed. (a3) MiB-1 staining is strong and extends throughout the full thickness of the epithelium. (a4) The ProEx C signal is weak but also covers the full thickness of the epithelium (original magnification × 400). (b1) A case diagnosed as SIL (LSIL) by only one of the reviewers (1/3). All stain signals occur in more than 25% lesional cells. (b2) The p16 signal is weak and patchy or focally distributed. (b3) The MiB-1 signal is strong and extends throughout the full thickness of the epithelium. (b4) The ProEx C signal is also strong and extends throughout the full thickness of the epithelium. In comparison with MIB-1, the ProEx C signal is stronger and seen in a larger number of cells (original magnification × 200). (c1) A case classified as HSIL by 3/3 reviewers. (c2) The p16 signal is diffuse, weak, and seen in the nucleus and cytoplasm. (c3) The MiB-1 signal is strong and present in less than 40% cells. (c4) The ProEx C signal is also strong and present in more than 90% cells (original magnification × 400).
Immature metaplastic epithelium with variable degrees of atypia (three cases, amplified fields of 400 × photomicrographs). (a1) Reparative squamous metaplastic epithelium diagnosed as NoSIL by all three reviewers. (a2) The p16 stain was considered negative due to the absence of nuclear staining. (a3) Immunoreactivity for MiB-1 is present within the middle one-third of the epithelium and considered positive. (a4) ProEx C is negative due to the absence of nuclear staining above the lower one-third of the epithelium. (b1) A case interpreted as SIL by 2/3 reviewers. (b2) The p16 stain is clearly negative. (b3) MiB-1 is positive, with staining seen throughout the full thickness of the epithelium. (b4) ProEx C is positive, with staining throughout the full thickness of the epithelium. (c1) Immature metaplastic squamous epithelium diagnosed as NoSIL by all three reviewers. (c2) P16 was considered positive. (c3) The ProEx C signal was considered negative because it was mostly confined to the lower one-third of the epithelium.
Note: An attempt to test HPV by PCR in a subset of cases was made. Amplification failed due to the small amount of residual tissue available for DNA extraction after serial levels of the paraffin block (data not shown).
Discussion
The fair-to-moderate diagnostic agreement among the three observers based on H&E alone was perhaps higher than expected, given that the cases were intentionally selected to be especially challenging. We focused on a subset of histological changes that had been subjected to p16 and/or MiB-1 immunostaining by the original pathologist due to a presumed question in classification. All cases were originally diagnosed as HSIL or reactive/atrophic epithelium during routine practice, based on H&E-stained sections with p16 or p16 plus MiB-1 immunostaining. During the slide review process, a small number of grading discrepancies surfaced among the reviewers. Although the majority (95% or more) of cases were corroborated as either reactive/changes or HSIL on review, a small percentage could not be readily distinguished from LSIL. Thus, for the purposes of this report, cases were classified as SIL and NoSIL. Interestingly, the ProEx C signal intensity in these occasional LSIL cases was typically stronger than with p16 and MiB-1 (Figure 3b), corroborating the results of a recent study.10 Focusing on the detection of LSIL, Shi et al, explored the role of the same biomarkers as in this study and found that compared with MiB-1 (85.3%) and p16 (76.5%) ProEx C not only had a higher sensitivity (94.1%), but also showed a stronger and more diffuse nuclear staining for LSIL cases. In the present study, we observed a larger number of cells stained by ProEx C in comparison with MiB-1 in both HSIL and LSIL cases (Figure 3).
Several antibodies to cell cycle proteins have been proposed for screening and diagnosis of HSIL.6, 12, 13, 14, 15 ProEx C has been proposed as a marker with a potential to detect HSIL in cervical biopsy specimens10 and liquid-based cervical cytology.16, 17 To date, only one other study has investigated the sensitivity and specificity of ProEx C in histopathological specimens.10 As mentioned above, the authors focused on LSILs, and included a limited sample of HSILs (14 cases) and benign squamous mucosa (14 cases). They found a sensitivity of 78.6% for HSIL detection; the specificity in their series was 100% for HSILs. In another study,17 ProEx C detected 10/10 HSILs and 0/10 negative for intraepithelial neoplasia or malignancy cases in SurePath® processed cytological samples, but the limited number of cases precluded extrapolating these results to practice. Kelly et al16 had a similar experience with ProEx C, recording a sensitivity of 85.3% and a specificity of 71.7%, by using biopsy-proven HSIL as an end point in a series of 317 SurePath® cytological samples. In our series, we found a sensitivity of 87% and a specificity of 71% for the ProEx C antibody in biopsy material.
It is important to emphasize that the gold standard in our study was a majority H&E-based diagnosis, an approach that lowers but does not eliminate classification error. Thus, some p16-negative cases that were considered lesions by H&E interpretation could in fact be reactive, albeit atypical, epithelium (Figure 4b). In this subset of cases, the ProEx C-positive result could be either interpreted as a false-positive result or as emblematic of a histological classification error. Six other cases diagnosed as SIL by H&E consensus were negative for p16, but positive for ProEx C. In only one of them, MiB-1 data were available and were positive as well. Interestingly, all of them presented with non-unanimous consensus opinion (2/3 votes for SIL). One was classified as LSIL, whereas the remaining five were classified as HSILs adjacent to a reactive and inflammatory process. Figure 4c is an example of a diagnostically problematic immature metaplasia with mild atypia. All three reviewers classified this case as NoSIL. P16 immunostaining was strong, whereas ProEx C was negative. Similarly, five cases classified by majority opinion as NoSIL were p16-positive and ProEx C-negative. These cases raise the possibility of false-positive p16 immunostaining, which was considered in three cases following review.
MiB-1 has been extensively tested in cervical lesions, and some reports have suggested that quantitative MiB-1 assessment could provide information about the progression risk of LSIL and cervical intraepithelial neoplasia grade 2.18, 19, 20, 21 Kelly et al16 raised the possibility that LSILs could be separated in two distinct groups based on their ProEx C positivity, with the positive group being more similar to HSIL in its biological behavior. The similarity between the signal distribution patterns of MiB-1 and ProEx C observed in our cases, as well as the possibility raised by this previous study in cytological samples, may justify future studies quantifying ProEx C and comparing the findings with HSIL outcome in follow-up.
The fact that p16/ProEx C predicted more NoSIL than p16/MiB-1 (Table 1), plus the close similarity between MiB-1 and ProEx C signal patterns, raises the possibility that ProEx C could be more efficient in distinguishing reactive epithelial changes from SIL than MiB-1. Reactive epithelia pose a problem of considerable significance for MiB-1 staining, which often extends through most of the reactive epithelium. The small subset of these cases, however, did not allow us to draw any conclusions with respect to the specificity of ProEx C compared with MiB-1.
In summary, ProEx C exhibits a high level of both sensitivity and specificity for distinguishing HSIL from its mimics. Its combination with p16 may be a better discriminator than p16/MiB-1 for triaging diagnostically difficult atypias in which the differential diagnosis includes HSIL.
References
Park TW, Richart RM, Sun XW, et al. Association between human papillomavirus type and clonal status of cervical squamous intraepithelial lesions. J Natl Cancer Inst 1996;88:355–358.
Crum CP, Mitao M, Levine RU, et al. Cervical papillomaviruses segregate within morphologically distinct precancerous lesions. J Virol 1985;54:675–681.
Genest DR, Stein L, Cibas E, et al. A binary (Bethesda) system for classifying cervical cancer precursors: criteria, reproducibility, and viral correlates. Hum Pathol 1993;24:730–736.
Stoler MH, Schiffman M, Atypical Squamous Cells of Undetermined Significance-Low-grade Squamous Intraepithelial Lesion Triage Study (ALTS) Group. Interobserver reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL Triage Study. JAMA 2001;285:1500–1505.
Crum CP, Rose PG . Cervical squamous neoplasia. In: Crum CP, Lee KR (eds). Diagnostic Gynecologic and Obstetric Pathology, 1st edn. Elsevier Saunders: Philadelphia, PA, 2006, pp 306–310.
Keating JT, Cviko A, Riethdorf S, et al. Ki-67, cyclin E, and p16INK4 are complimentary surrogate biomarkers for human papilloma virus-related cervical neoplasia. Am J Surg Pathol 2001;25:884–891.
Chen Y, Miller C, Mosher R, et al. Identification of cervical cancer markers by cDNA and tissue microarrays. Cancer Res 2003;63:1927–1935.
Shi SR, Key ME, Kalra KL . Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem 1991;39:741–748.
Klaes R, Benner A, Friedrich T, et al. p16INK4a immunohistochemistry improves interobserver agreement in the diagnosis of cervical intraepithelial neoplasia. Am J Surg Pathol 2002;26:1389–1399.
Shi J, Liu H, Wilkerson M, et al. Evaluation of p16INK4a, minichromosome maintenance protein 2, DNA topoisomerase IIalpha, ProE(X) C, and p16INK4a/ProE(X) C in cervical squamous intraepithelial lesions. Hum Pathol 2007;38:1335–1344.
DeLong ER, DeLong DM, Clarke-Pearson DL . Comparing the areas under two or more correlated receiver operating curves: a nonparametric approach. Biometrics 1988;44:837–845.
Lorenzato M, Caudroy S, Bronner C, et al. Cell cycle and/or proliferation markers: what is the best method to discriminate cervical high-grade lesions? Hum Pathol 2005;36:1101–1107.
Akpolat I, Smith DA, Ramzy I, et al. The utility of p16INK4a and Ki-67 staining on cell blocks prepared from residual thin-layer cervicovaginal material. Cancer 2004;102:142–149.
Sahebali S, Depuydt CE, Boulet GA, et al. Immunocytochemistry in liquid-based cervical cytology: analysis of clinical use following a cross-sectional study. Int J Cancer 2006;118:1254–1260.
Moore GD, Lear SC, Wills-Frank LA, et al. Differential expression of cdk inhibitors p16, p21cip1, p27kip1, and cyclin E in cervical cytological smears prepared by the ThinPrep method. Diagn Cytopathol 2005;32:82–87.
Kelly D, Kincaid E, Fansler Z, et al. Detection of cervical high-grade squamous intraepithelial lesions from cytologic samples using a novel immunocytochemical assay (ProEx C). Cancer 2006;108:494–500.
Shroyer KR, Homer P, Heinz D, et al. Validation of a novel immunocytochemical assay for topoisomerase II-alpha and minichromosome maintenance protein 2 expression in cervical cytology. Cancer 2006;108:324–330.
Goel MM, Mehrotra A, Singh U, et al. MIB-1 and PCNA immunostaining as a diagnostic adjunct to cervical Pap smear. Diagn Cytopathol 2005;33:15–19.
Kruse AJ, Baak JP, Janssen EA, et al. Ki67 predicts progression in early CIN: validation of a multivariate progression-risk model. Cell Oncol 2004;26:13–20.
Kruse AJ, Baak JP, Janssen EA, et al. Low- and high-risk CIN 1 and 2 lesions: prospective predictive value of grade, HPV, and Ki-67 immuno-quantitative variables. J Pathol 2003;199:462–470.
Kruse AJ, Skaland I, Janssen EA, et al. Quantitative molecular parameters to identify low-risk and high-risk early CIN lesions: role of markers of proliferative activity and differentiation and Rb availability. Int J Gynecol Pathol 2004;23:100–109.
Acknowledgements
We acknowledge Chandler Birch and Elin Agoston for their technical support during the period of the research. AP Pinto is a recipient of a postdoctoral scholarship from the National Council of Technological and Scientific Development (CNPq) (Brazil).
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper was presented in part at the United States and Canadian Academy of Pathology (USCAP) Annual Meeting, Denver, CO, March 1–7, 2008.
Disclosure/conflict of interest
The authors of this manuscript do not have a financial relationship with the commercial enterprises whose products are discussed in this manuscript.
Rights and permissions
About this article
Cite this article
Pinto, A., Schlecht, N., Y C Woo, T. et al. Biomarker (ProExTM C, p16INK4A, and MiB-1) distinction of high-grade squamous intraepithelial lesion from its mimics. Mod Pathol 21, 1067–1074 (2008). https://doi.org/10.1038/modpathol.2008.101
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2008.101
Keywords
This article is cited by
-
HPV-Type Distribution and Reproducibility of Histological Diagnosis in Cervical Neoplasia in Poland
Pathology & Oncology Research (2015)
-
Präkanzeröse Veränderungen der Zervix
Der Pathologe (2011)