Abstract
NLRP6 is a member of the Nod-like receptor family, whose members are involved in the recognition of microbes and/or tissue injury. NLRP6 was previously demonstrated to regulate the production of interleukin (IL)-18 and is important for protecting mice against chemically induced intestinal injury and colitis-associated colon cancer. However, the cellular mechanisms by which NLRP6 reduces susceptibility to colonic inflammation remain unclear. Here, we determined that NLRP6 expression is specifically upregulated in Ly6Chi inflammatory monocytes that infiltrate into the colon during dextran sulfate sodium (DSS)-induced inflammation. Adoptive transfer of wild-type (WT) Ly6Chi inflammatory monocytes into Nlrp6−/− mice was sufficient to protect them from mortality, significantly reducing intestinal permeability and damage. NLRP6-deficient inflammatory monocytes were defective in tumor necrosis factor α (TNFα) production, which was important for reducing DSS-induced mortality and was dependent on autocrine IL-18 signaling by inflammatory monocytes. Our data reveal a previously unappreciated role for NLRP6 in inflammatory monocytes, which are recruited after DSS-induced intestinal injury to promote barrier function and limit bacteria-driven inflammation. This study highlights the importance of early cytokine responses, particularly NLRP6-dependent and IL-18-dependent TNFα production, in preventing chronic dysregulated inflammation.
Similar content being viewed by others
Introduction
Inflammatory bowel disease (IBD) is a major health problem that results in significant morbidity and mortality due to recurrent episodes of bloody diarrhea, weight loss, and complications from chronic intestinal inflammation, such as cancer. Current approved therapies for IBD rely primarily on symptom management and inhibiting inflammation, but do not address the underlying mechanism behind IBD pathogenesis. The current prevailing model for the pathogenesis of IBD is the development of an imbalance in the microbial community structure within the intestine, or dysbiosis, and/or aberrant immune responses to commensal bacteria in a genetically susceptible host.1 A better understanding of events that lead to dysregulated inflammation is needed to identify targets for treatment and cure.
Recently, the Nod-like receptor (NLR), NLRP6, has been shown to be important for promoting intestinal homeostasis and for protection against the development of colitis and colitis-associated tumorigenesis in mice.1, 2, 3, 4 The mechanism by which NLRP6 protects against colitis remains to be fully elucidated. Gene ontogeny analyses have established a link between NLRP6 and intestinal epithelial cell (IEC) repair.4 NLRP6 also regulates interleukin (IL)-18 production, which is important for epithelial repair and protection against inflammation-associated colon tumorigenesis.1, 2, 4, 5, 6 NLRP6 is highly expressed in IECs, and bone marrow (BM) chimera experiments demonstrated that IL-18 production by epithelial cells is important for reduced susceptibility to dextran sulfate sodium (DSS)-induced intestinal injury and inflammation, suggesting that NLRP6 functions primarily in epithelial cells to promote IL-18 production and epithelial repair. NLRP6 also regulates autophagy within intestinal goblet cells to affect mucus secretion, which contributes to intestinal homeostasis.7 However, NLRP6 is also expressed in hematopoietic cell populations, and we have previously demonstrated that NLRP6 functions in BM-derived cells to limit colitis-associated tumorigenesis.1 Thus, the specific cell type that is important for NLRP6-mediated protection against the development of colitis and tumorigenesis has not been definitively identified.
In this study, we examined the expression of NLRP6 in various hematopoietic cell populations within the lamina propria (LP) during DSS-induced intestinal injury and determined that inflammatory monocytes, defined as CD11b+Ly6GintLy6Chi cells, induce NRLP6 expression in response to DSS. More importantly, the adoptive transfer of wild-type (WT) Ly6Chi monocytes into Nlrp6−/− mice improves survival after DSS treatment. NLRP6-deficient Ly6Chi monocytes had impaired production of tumor necrosis factor α (TNFα) and reactive oxygen species (ROS), and the injection of recombinant TNFα (rTNFα) into Nlrp6−/− mice early during DSS treatment was sufficient for protection against mortality. We further demonstrate that Il18−/− and Il18r1−/− Ly6Chi inflammatory monocytes have similar defects in TNFα production, and their adoptive transfer into Nlrp6−/− mice failed to rescue DSS-induced mortality, suggesting that autocrine IL-18 signaling by inflammatory monocytes is important for TNFα production and protection against acute intestinal injury. Altogether, these studies reveal a protective role for early TNFα production by inflammatory monocytes, which is at least, in part, IL-18- and NRLP6-dependent and is critical for limiting dysregulated commensal-driven intestinal inflammation.
Results
NLRP6 function in Ly6Chi inflammatory monocytes reduces susceptibility to DSS-induced intestinal injury
We previously demonstrated that Nlrp6−/− mice are more susceptible to DSS-induced colitis as well as colitis-associated tumorigenesis after treatment with the carcinogen azoxymethane (AOM) and DSS.1, 2, 4 Furthermore, NLRP6 activity in BM-derived cells was important for limiting inflammation-associated tumors.1 To identify the cell type responsible for the protective effects of NLRP6, expression of NLRP6 was measured in different cell populations in the BM and colon LP before and after DSS treatment. We analyzed NLRP6 mRNA expression in IECs, intraepithelial lymphocytes (IELs), BM, and LP cells from WT mice on day 0 and day 10 (at the end of 5 days of 2% DSS) in the AOM/DSS model of colitis-associated tumorigenesis.1 We determined that NLRP6 expression was upregulated in the LP, but not IEC, IEL, or BM cells in response to DSS (Figure 1a). Within the LP, NLRP6 was specifically increased in myeloid cells, and in particular, Ly6Chi monocytes and neutrophils, after DSS treatment with the highest induction in Ly6Chi inflammatory monocytes (Figure 1b). In contrast, NLRP6 expression did not change in T cells and was undetectable in B cells (Figure 1b). We confirmed the upregulation of NLRP6 expression in LP cells and myeloid cells within the LP in WT mice treated with DSS only, indicating that the observed change in NLRP6 expression is not dependent on AOM (Supplementary Figure 1 online).
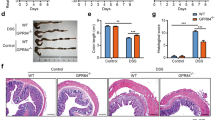
NLRP6 is induced in lamina propria Ly6Chi monocytes during dextran sulfate sodium (DSS)-induced inflammation, and is important for reducing susceptibility to colitis. (a) NLRP6 levels were measured in bone marrow (BM), lamina propria (LP), intestinal epithelial cells, and intraepithelial lymphocytes of wild-type (WT) mice at day 0 and day 10 of azoxymethane (AOM)/DSS by qPCR. (b) NLRP6 expression was measured in CD3+B220-CD11b− T cells, CD3-B220+CD11b− B cells, CD3−CD11b+Ly6ChiLy6G- monocytes and CD3−CD11b+Ly6CintLy6G+ neutrophils within the BM and LP. Data are representative of three independent experiments; n=11 for day 0, n=10 for day 10. *P<0.05, **P<0.001, respectively, as compared with day 0 time point of the same genotype. (c) Representative plots of Ly6C vs. Ly6G staining of CD11b+ BM cells (top). Kaplan–Meier survival curves of mice treated with 7 days of 3.5% DSS (bottom). (d) Percent weight change with 3.5% DSS administration. Data are representative of two independent experiments; n=15, n=24, n=14 for WT, Nlrp6−/− and Nlrp6−/−+WT Ly6Chi monocytes groups respectively. *P<0.05, **P<0.001, respectively, as compared with Nlrp6−/−.
Upregulation of NLRP6 in response to DSS in Ly6Chi inflammatory monocytes prompted us to investigate if NLRP6 function in this population of cells was important for maintaining intestinal homeostasis. WT Ly6Chi monocytes (CD3−CD11b+Ly6ChiLy6G−) were fluorescence-activated cell sorting (FACS)-sorted to ∼99% purity (Figure 1c), and were adoptively transferred into Nlrp6−/− mice on day 3.5 of a 7-day course of high-dose DSS (3.5%). In contrast to mock-treated Nlrp6−/− mice, which had a 15% survival rate after DSS treatment, Nlrp6−/− mice that received Ly6Chi monocytes were protected from lethality with a survival rate of ∼70% (Figure 1c). Consistent with improved survival, Nlrp6−/− mice harboring WT Ly6Chi monocytes also showed significantly less weight loss (Figure 1d). These results strongly suggest that NLRP6 is upregulated in inflammatory monocytes that are important for reducing DSS-induced mortality. To demonstrate that donor Ly6Chi monocytes can be recruited to the intestine during DSS treatment, we adoptively transferred Ly6Chi monocytes isolated from transgenic mice that express green fluorescent protein (GFP) into Nlrp6−/− mice during DSS treatment, and detected GFP+ Ly6Chi monocytes in the LP of recipient mice (Supplementary Figure 2a). As confirmation that the recruitment of inflammatory monocytes into the intestinal LP is important for NLRP6-mediated protection against DSS-induced injury and inflammation, we adoptively transferred Nlrp6−/− mice with Ly6Chi monocytes isolated from mice deficient in CCR2, which is expressed by inflammatory monocytes and is required for their recruitment to sites of inflammation,8 and observed no improvement in weights or survival with DSS treatment (Supplementary Figure 2b–d).
Ly6Chi monocytes can be further characterized by their CX3CR1 expression. During intestinal inflammation, Ly6ChiCX3CR1int inflammatory monocytes are recruited to the LP.9 Consistently after 5 days of DSS, all Ly6Chi monocytes in the LP of WT and Nlrp6−/− mice express CX3CR1 (Supplementary Figure 3a). To determine CX3CR1 expression in Ly6Chi donor monocytes in adoptive transfer experiments and track their recruitment in the intestine during DSS treatment, we used CX3CR1-GFP mice, in which CX3CR1-expressing cells are labeled with GFP, as donors and Ccr2−/− mice as recipients. Ly6Chi donor monocytes were mostly CX3CR1int (∼80%), (Supplementary Figure 3b), but after transfer into Ccr2−/− mice treated with DSS, all donor CCR2+ cells in the LP were CX3CR1int (Supplementary Figure 3c). These results strongly suggest that Ly6Chi monocytes that migrate into the colon in this model and have protective effects in Nlrp6−/− mice represent a relatively homogenous population of CX3CR1int inflammatory monocytes.
WT Ly6Chi monocytes limit tumor growth in Nlrp6−/− mice
We had previously reported that Nlrp6−/− mice developed more colon tumors compared with WT mice in the AOM/DSS model of inflammation-associated tumorigenesis.1 To determine whether NLRP6 function in inflammatory monocytes is important for reducing inflammation-associated tumorigenesis, we adoptively transferred WT Ly6Chi monocytes into Nlrp6−/− mice 3.5 days after the start of each cycle of 2% DSS (Figure 2a). As previously published, Nlrp6−/− mice developed more tumors compared with WT mice (Figure 2b).1 Adoptive transfer of WT Ly6Chi monocytes resulted in fewer tumors; however, this did not reach statistical significance (Figure 2b). The transfer of WT Ly6Chi monocytes into Nlrp6−/− mice did result in a significant reduction in tumor sizes with fewer tumors greater than 2 mm in size compared with that in Nlrp6−/− mice (Figure 2c), and reduced weight loss early during AOM/DSS treatment (days 14–26, Figure 2d).
Adoptive transfer of wild-type (WT) Ly6Chi monocytes into Nlrp6−/− mice significantly reduces tumor growth in azoxymethane (AOM)/DSS (dextran sulfate sodium) model of colitis-associated tumorigenesis. (a) Age- and sex-matched Nlrp6−/− and WT mice were treated with AOM followed by three 5-day cycles of 2% DSS. Sorted Ly6Chi monocytes were injected i.v. 3.5 days after the start of each DSS cycle. Three weeks after the last cycle of DSS mice were sacrificed and tumors grossly counted with a stereomicroscope. Number (b) and size (c) of tumors are shown. (d) Percent weight change during AOM/DSS treatment. Data are representative of three independent experiments, n=15, n=10, n=19, n=22 for WT, WT+WT Ly6Chi monocytes, Nlrp6−/− and Nlrp6−/−+WT Ly6Chi monocytes groups respectively. *P<0.05, **P<0.001, respectively, as compared with WT (b) or Nlrp6−/− mice (d).
NLRP6 function in Ly6Chi monocytes promotes resistance against DSS-induced damage.
To determine the effects of WT Ly6Chi monocytes within the colon of Nlrp6−/− mice during the acute inflammatory response to DSS, we looked specifically on day 10 of the AOM/DSS model of tumorigenesis when mice have completed 5 days of 2% DSS before the onset of chronic, dysregulated inflammation in Nlrp6−/− mice.1 Increased intestinal permeability is a classical hallmark of DSS-induced IEC damage.1, 2, 4 To determine if the adoptive transfer of WT Ly6Chi monocytes affected intestinal permeability, mice were gavaged with FITC-dextran and its passage into the peripheral circulation was measured. As demonstrated previously,1 naive WT and Nlrp6−/− mice had very low levels of serum fluorescence indicative of an intact epithelial barrier, whereas DSS-treated mice, especially Nlrp6−/− mice, had higher levels of serum fluorescence suggesting increased intestinal permeability (Figure 3a).1 Importantly, the intestinal permeability of Nlrp6−/− mice adoptively transferred with WT Ly6Chi monocytes was similar to that of WT mice after exposure to DSS, and was significantly reduced compared with that of mock-treated Nlrp6−/− mice (Figure 3a). Consistently, there was a reduction in bacterial translocation into the mesenteric lymph nodes (MLNs) as measured by qPCR in Nlrp6−/− mice adoptively transferred with WT Ly6Chi monocytes (Figure 3b). Levels of fecal lipocalin-2, a surrogate marker of intestinal epithelial damage,10 were also reduced in Nlrp6−/− mice adoptively transferred with WT Ly6Chi monocytes (Figure 3c). Histological scoring of colitis demonstrated reduced intestinal epithelial damage and inflammation in the presence WT Ly6Chi monocytes (Figure 3d). Thus, NLRP6 activity in Ly6Chi inflammatory monocytes is important in limiting DSS-induced damage.
Adoptive transfer of wild-type (WT) Ly6Chi monocytes into Nlrp6−/− mice limits bacterial translocation and intestinal damage. Age- and sex-matched Nlrp6−/− and WT mice were treated with azoxymethane (AOM) followed by 5 days of 2% dextran sulfate sodium (DSS). WT Ly6Chi monocytes were adoptively transferred into Nlrp6−/− mice 3.5 days after start of DSS. (a) Mice were gavaged FITC-dextran at end of 5 days of DSS followed by serum collection and measurement of fluorescence 4 h later. (b) Normalized levels of total bacteria/mesenteric lymph node as measured by quantitative PCR after 5 days of 2% DSS. (c) Fecal lipocalin-2 levels as measured by ELISA (enzyme-linked immunosorbent assay) after 5 days of DSS. (d) Histological inflammatory scores based on extent of inflammatory cell infiltration and intestinal epithelial cell damage; Micrographs of H&E sections are at 200 × magnification. Arrow points to focal erosion with inflammatory infiltrate. Bracket indicates ulcerated epithelium with inflammatory infiltrate in lamina propria and submucosa. Data are representative of three independent experiments, n=15, n=16, n=20 for WT, Nlrp6−/− and Nlrp6−/−+WT Ly6Chi monocytes groups respectively. *P<0.05, **P<0.001, respectively, as compared with day 0 of both genotypes (a), or as compared with Nlrp6−/− (b,c) or as compared with WT (d).
Nlrp6−/− Ly6Chi inflammatory monocytes have impaired TNFα and ROS production
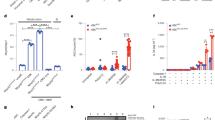
Inflammatory Ly6Chi monocytes perform critical functions during inflammation, including phagocytosis, production of ROS, and secretion of cytokines.11 These functions limit bacterial translocation, promote tissue repair, and ultimately, help to resolve inflammation. To determine whether Nlrp6−/− Ly6Chi inflammatory monocytes have a defect in phagocytosis, Ly6Chi monocytes were isolated from naive and AOM/DSS-treated WT and Nlrp6−/− mice (day 10).1 In agreement with previous studies, we observed no defect in phagocytosis as BM-sorted Ly6Chi monocytes from WT and Nlrp6−/− mice exhibited equivalent levels of internalized GFP+ Escherichia coli (Figure 4a).12 However, ROS production was significantly reduced in BM-derived and LP-isolated Nlrp6−/− Ly6Chi monocytes as compared with WT Ly6Chi monocytes after DSS treatment (Figure 4b,c). We next measured levels of various cytokines produced by LP inflammatory Ly6Chi monocytes isolated from AOM/DSS-treated Nlrp6−/− mice and cultured overnight. Nlrp6−/− LP-derived inflammatory monocytes exhibited decreased early production of TNFα at the completion of DSS, whereas other cytokines such as IL-6 and IL-1β were produced at levels similar to that by WT Ly6Chi monocytes (Figure 4d). Consistently, TNFα mRNA expression was also significantly reduced in Nlrp6−/− LP Ly6Chi monocytes (Figure 4e). By day 16, however, approximately 1 week after completion of DSS, TNFα levels were no longer significantly different between WT and Nlrp6−/− inflammatory monocytes, suggesting that NLRP6-independent pathways may be compensating (Figure 4d). The defect in TNFα production was specific to Nlrp6−/− inflammatory monocytes as Nlrp6−/− resident LP macrophages, defined as CD11b+Ly6Clow/-F4/80highLy6G− cells,9 exhibited no differences in cytokine production compared with WT (Supplementary Figure 4). In mice treated with 5 days of DSS alone, we observed similar defects in TNFα production, but not IL-1β, by Nlrp6−/− Ly6Chi monocytes associated with increased fecal lipocalin levels in Nlrp6−/− mice (Supplementary Figure 5).
NLRP6-deficient Ly6Chi monocytes have reduced ROS and TNFα production during the acute inflammatory response to dextran sulfate sodium (DSS). (a) Ly6Chi monocytes were sorted from WT or Nlrp6−/− mice and incubated with GFP-labeled E. coli at MOI 0, 5, 25, and 60. Phagocytosis was measured by percent GFP+ Ly6Chi monocytes. Data are representative of two independent experiments, n=6. ROS production (MFI) as measured by CellROX deep red in bone marrow- (b) or lamina propria (LP)-derived (c) Ly6Chi monocytes at the indicated time points. Ly6Chi monocytes from WT or Nlrp6−/− mice were sorted from the LP after 5 days of 2% DSS (day 10, AOM/DSS model) or 6 days after completion of DSS (day 16, AOM/DSS model) and cytokine levels were measured by ELISA (enzyme-linked immunosorbent assay) (d) and by qPCR (e). Data are representative of at least three independent experiments, mean±s.e.m.; n=14/genotype, (day 10); n=12/genotype (day 0); n=8/genotype (day 16). *P<0.05, as compared with WT.
To determine if NLRP6 upregulation is associated with TNFα production, we sorted Ly6Chi monocytes from the BM of WT mice, and stimulated them with rosiglitazone, which was previously demonstrated to increase NLRP6 expression in colon epithelial cells.13 We indeed observed upregulation of NLRP6 expression when WT monocytes were treated with rosiglitazone (Supplementary Figure 6a) along with increased TNFα production in WT macrophages, which did not occur to the same extent in Nlrp6−/− Ly6Chi monocytes (Supplementary Figure 6b), suggesting that the TNFα response is indeed partially NLRP6 dependent. We next determined whether a defect in the recruitment of Nlrp6−/− inflammatory monocytes into the LP was associated with decreased TNFα production. However, examination of monocyte populations in the LP of WT and Nlrp6−/− mice on day 0 and day 10 after completion of 5 days of DSS showed, in fact, increased numbers of Ly6Chi monocytes recruited to the LP of Nlrp6−/− mice (Supplementary Figure 7). To determine if the impairment in TNFα production by Nlrp6−/− Ly6Chi monocytes was not a result of global deficiency in cytokine responses, we isolated inflammatory Ly6Chi monocytes from the LP of WT and Nlrp6−/− mice on day 10 of AOM/DSS treatment and performed gene expression analysis by microarray. Functional pathway analysis revealed increased mRNA expression of several cytokines and chemokines in Nlrp6−/− Ly6Chi cells, while TNFα was one of the few cytokines that was the most reduced (Supplementary Figure 8).
Early production of TNFα is important for resistance against DSS-induced intestinal injury
Elevated levels of TNFα have frequently been associated with disease severity in chronic IBD patients;14 however, TNFα also promotes epithelial restitution after DSS-induced injury.15 To determine whether the early production of TNFα by inflammatory monocytes is important for protection against intestinal damage and DSS-induced mortality, Nlrp6−/− mice were injected with rTNFα on days 3–7 during a 7-day course of high dose 3.5% DSS. As expected, the administration of rTNFα into WT mice had no effect on their survival (Figures 5a,b). In contrast, Nlrp6−/− mice that received rTNFα showed significant improvement in survival with decreased weight loss compared with Nlrp6−/− mice that did not receive supplemental rTNFα. There was reduced colonic inflammation and damage as suggested by decreased lipocalin-2 levels as well as less bacterial translocation into the MLNs in Nlrp6−/− mice that received rTNFα (Figure 5c,d). Finally, histologic examination of colons revealed a significant improvement in inflammatory scores for Nlrp6−/− mice that received rTNFα after 5 days of 2% DSS (day 10 AOM/DSS model), reflecting reduced levels of epithelial damage, hyperplasia, and inflammation (Figure 5e). Altogether, these results suggest that NLRP6-dependent production of TNFα by WT inflammatory monocytes is important for limiting intestinal damage and bacterial translocation, thereby reducing DSS-induced inflammation and mortality.
Recombinant TNFα administration into Nlrp6−/− mice early during dextran sulfate sodium (DSS) treatment reduces susceptibility to DSS-induced colitis. (a) Kaplan–Meier survival curves and (b) percent weight change of age- and sex-matched WT and Nlrp6−/− mice treated with 3.5% DSS for 7days with or without rTNFα injections given on days 3–7. Data are representative of two independent experiments, mean±s.e.m.; n=15, n=16 for Nlrp6−/− and Nlrp6−/−+1μg/mouse rTNFα respectively; n=5 for WT and WT+1 μg per mouse rTNFα (c) Fecal lipocalin-2 levels measured in WT and Nlrp6−/− mice (day 10, azoxymethane (AOM)/DSS model) after adoptive transfer of Ly6Chi monocytes or 1 μg TNF given on days 3–5 of DSS (d) Normalized levels of total bacteria/mesenteric lymph node as measured by qPCR after 5 days of 2% DSS (day 10 AOM/DSS model). (e) H&E micrographs at 100 × after 5 days of 2% DSS (top) with histological scoring are shown. Bracket indicates diffuse ulceration with inflammatory infiltrate extending throughout the lamina propria and submucosa; n=5, n=6, n=6, n=7 for WT, Nlrp6−/−, Nlrp6−/−+1 μg per mouse rTNFα, and Nlrp6−/−+WT Ly6Chi monocytes groups respectively.
Production of TNFα by Ly6Chi inflammatory monocytes is dependent on IL-18 signaling
We have previously demonstrated that Nlrp6−/− mice have impaired production of mature IL-18, which is important for epithelial repair to limit commensal-driven inflammation.1 Although it was previously shown that IL-18 is predominantly produced by the intestinal epithelium,3 LP cells are also capable of secreting IL-18.16 Importantly, we also observed reduced levels of IL-18 production by Ly6hi inflammatory monocytes isolated from DSS-treated Nlrp6−/− mice compared with that from WT (Supplementary Figure 9). To determine if IL-18 production by Ly6Chi monocytes is important for resistance to DSS-induced mortality, Nlrp6−/− mice were adoptively transferred with Il18−/− Ly6Chi monocytes, and survival of these mice was compared with that of mock-transferred Nlrp6−/− and WT mice after 7 days of 3.5% DSS (Figure 6a). Nlrp6−/− mice that received Il18−/− monocytes had similar levels of mortality and weight loss compared with Nlrp6−/− mice (Figures 6a and b). Interestingly, Ly6Chi monocytes isolated from DSS-treated Il18−/− mice also had impaired production of TNFα, but not IL-1β, indicating that TNFα production by Ly6Chi monocytes is, in part, IL-18 dependent (Figure 6c).
Autocrine IL-18 production by Ly6Chi monocytes protects against dextran sulfate sodium (DSS)-induced colitis by increasing TNFα production. Ly6Chi monocytes were purified from WT, Il18−/− or Il18r1−/− mice and i.v. injected (2 × 106 cells/per mouse) into Nlrp6−/− recipients at day 3.5 of a 7-day course of 3.5% DSS. Mice survival (a) and weight loss (b) for WT, Nlrp6−/− and Nlrp6−/−+Il18−/− Ly6Chi monocytes was assessed. n=8 per group (c) Ly6Chi monocytes were sorted from the lamina propria after 5 days of 2% DSS (day 10 azoxymethane (AOM)/DSS model) from Il18−/− mice, and cytokine levels in overnight cultures were measured by ELISA (enzyme-linked immunosorbent assay). n=4 per group. Mice survival (d) and weight loss (e) after adoptive transfer of Il18R−/− Ly6Chi monocytes into Nlpr6−/− mice. n=5 for WT, n=11 for Nlrp6−/− and n=8 for Nlrp6−/−+Il18r1−/− Ly6Chi monocytes. (f) Cytokine levels measured from culture supernatants of WT and Il18R−/− Ly6Chi monocytes after 5 days of 2% DSS (day 10 AOM/DSS model), n=4/group. *P<0.05 and **P<0.001.
To determine whether inflammatory monocytes respond to IL-18 to promote TNFα production and resistance to DSS, Nlrp6−/− mice were also adoptively transferred with Il-18R−/− monocytes and survival measured after treatment with high-dose DSS. Nlrp6−/− mice that received Il-18R−/− monocytes were equally susceptible to DSS-induced mortality and weight loss as mock-treated Nlrp6−/− mice (Figure 6d,e). Furthermore, cultures of Ly6Chi cells isolated from DSS-treated Il18r1−/− mice also exhibited a defect in TNFα production, while secretion of IL-1β was actually increased rather than decreased (Figure 6f). These data strongly suggest that autocrine IL-18 signaling by inflammatory monocytes is important for regulating TNFα production early during the acute inflammatory response to DSS to promote resistance to intestinal injury and lethality.
Discussion
NLRP6 is a member of the NLR family that is important for maintaining intestinal homeostasis.3 Our studies provide important insights into the mechanism by which NLRP6 protects against the development of colitis. We show that NLRP6 functions particularly in Ly6Chi inflammatory monocytes that are recruited to the intestinal LP during inflammation to protect the host against DSS-induced epithelial injury and subsequent life-threatening immunopathology. NLRP6 deficiency in inflammatory monocytes was also associated with defective TNFα production early in response to DSS, which, in turn, is important for reducing mortality. Our data further suggest that TNFα production is mediated by autocrine IL-18 signaling by Ly6Chi inflammatory monocytes. Therefore, we propose a model in which NLRP6 activation in inflammatory monocytes recruited in response to DSS-induced inflammation results in IL-18 secretion that upregulates their production of TNFα, which promotes epithelial repair and timely resolution of damage.15
Although IL-18 levels can be elevated in patients with active inflammatory disease,16, 17 studies in mice have also demonstrated the protective effects of IL-18 as I118−/− and Il18r1−/− mice are more susceptible to colitis and colitis-associated colon tumorigenesis.18 This discrepancy may reflect differential roles of IL-18 that is dependent on timing and context. The production of IL-18 early during DSS-induced inflammation is important for epithelial restitution and resolution of inflammation, but in the setting of chronic, dysregulated inflammation, excessive IL-18 production can exacerbate disease.19 Consistently, impairment in IL-18 production during DSS-induced colitis by NLRP6-deficient mice is observed only early on during and after DSS treatment before the development of significant intestinal damage and inflammation.1 Our data demonstrates that Nlrp6−/− Ly6Chi monocytes in the LP also have impaired early IL-18 production. However, it is not currently known whether the reduced IL-18 secretion is due to decreased inflammasome-mediated caspase-1 activation or reflects a defect in a non-canonical mechanism that remains to be identified. In addition, the mechanism for IL-18-mediated protection is unclear. Previous studies suggested that IL-18 is predominantly expressed by the epithelium and that epithelial-derived IL-18 is important for protection against DSS-induced damage.3, 20 IL-18 production is also associated with upregulation of IL-22, which is implicated in epithelial repair.21 Our study suggests an additional mechanism for IL-18 function, which is to upregulate TNFα production in inflammatory monocytes.
TNFα levels can be significantly elevated in IBD patients, and TNFα antagonism is an approved therapy for IBD. The excessive production of TNFα observed in patients with IBD and in mouse models of colitis likely reflects active disease that is already chronic and dysregulated. However, similar to IL-18, early production of TNFα is important for tissue repair and maintaining intestinal homeostasis.15, 22, 23, 24 Germ-free mice or mice that lack the adapter protein MyD88, which functions downstream of TLR-mediated bacterial sensing, have significant DSS-induced epithelial damage associated with the lack of induction of reparative cytokines, including TNFα.15, 25 In addition, TNFαNn addition, or mice that lack the adapter protein MyD88, which fun22, 24 Tnfr1−/− or Tnfr2−/− mice as well as Tnfα−/− mice also develop more severe DSS-induced colitis with elevated levels of pro-inflammatory cytokines, increased epithelial damage, and increased mortality as compared with WT mice.24, 26, 27, 28 Although there has been conflicting data that demonstrate a pathogenic role for TNFR2 in DSS-induced colitis,29 inflammatory monocytes are more likely to express TNFR1, while TNFR2 upregulation in colonic epithelial cells is observed in patients with inflammatory bowel disease.30, 31 Altogether, these studies are consistent with our data since Nlrp6−/− mice that receive rTNFα early during DSS treatment exhibit reduced intestinal inflammation and bacterial translocation.
We previously demonstrated that NLRP6 functions in a BM-derived cell to limit inflammation-associated tumorigenesis.1 In the current study, we show that NLRP6 was induced specifically in myeloid cells within the LP during DSS-induced colitis. Furthermore, NLRP6 activity in Ly6Chi monocytes is important for reducing susceptibility to DSS-induced colitis. Despite the protection against the detrimental effects of inflammation, we did not see a statistically significant reduction in the number of tumors. Rather, the size of tumors was significantly decreased, suggesting that NLRP6 function in monocytes limits inflammation that primarily drives tumor growth. Consistently, the downregulation of inflammatory responses by removal of NF-kB activity in myeloid cells also resulted in reduced tumor size rather than tumor number.32 However, it is also possible that the administration of Ly6Chi monocytes only once with each DSS cycle is insufficient to affect tumor number, or that another BM-derived cell in addition to monocytes is necessary for protection.
There are two major populations of monocytes in the murine intestinal LP, classical Ly6Chi inflammatory monocytes and Ly6C− monocytes that differentiate into macrophages.11 Ly6Chi monocytes can be further differentiated by their expression level of CX3CR1.33, 34 LP Ly6Chi monocytes can differentiate into CX3CRhi resident macrophages or effector CX3CR1int inflammatory monocytes whose primary function is to rapidly produce pro-inflammatory cytokines after their recruitment to sites of inflammation and injury. Our data strongly indicates that during DSS-induced inflammation, the majority of Ly6Chi monocytes that are recruited to the LP express CX3CR1 and adoptively transferred Ly6Chi inflammatory monocytes recruited to the intestinal LP are predominantly Ly6ChiCX3CR1int (Supplementary Figure 3), consistent with previous studies.35 Although inflammatory monocytes have been implicated in exacerbating colitis since they are capable of producing pro-inflammatory factors, and are found in increased numbers in persistently inflamed tissue,11, 36 they also have important roles in restoring homeostasis and limiting damage. This is largely through their ability to be early responders to tissue injury and produce cytokines, including TNFα and IL18 that have reparative functions thereby allowing epithelial restitution and resolution of inflammation.9 Consistently, depletion of intestinal monocytes in WT mice resulted in increased susceptibility to colitis.37 Our data further highlight a positive impact by Ly6Chi monocytes via NLRP6 as an early source of TNFα to prevent uncontrolled inflammation in response to epithelial damage. NLRP6 deficiency in Ly6Chi monocytes is also associated with defective ROS production, which may further limit translocation of bacteria and exacerbation of intestinal inflammation.38 Indeed, the adoptive transfer of Ly6Chi monocytes resulted in reduced bacterial translocation and improved barrier function. It is possible that the decreased ROS production is related to defective production of TNFα, which is needed to promote the assembly of the NADPH oxidase complex that produces ROS.39 Nonetheless, these results are somewhat surprising in light of the study by Anand et al., which suggests that NLRP6 negatively regulates cytokine responses, including TNFα, in BM-derived macrophages (BMDMs) after exposure to bacterial ligands.12 One possible explanation for this disparate finding is that BMDMs cultured in vitro represent a population distinct from inflammatory monocytes. In fact, we demonstrate that not all monocyte populations regulate TNFα production via NLRP6 as we observed no difference in TNFα production between WT and Nlrp6−/− LP resident macrophages. Thus, it is likely that differential TNFα responses reflect not only differences in the particular cell population and location, but also the context in which inflammation occurs.
How NLRP6 promotes TNFα production in inflammatory monocytes remains to be fully elucidated. The lack of a known NLRP6 receptor agonist has made the identification of cognate signaling pathways challenging. However, our data suggest that the mechanism may be mediated indirectly through the regulation of IL-18 production in monocytes. The adoptive transfer of Il18−/− or Il18r1−/− monocytes into Nlrp6−/− mice failed to rescue DSS-induced mortality, suggesting that autocrine IL-18 signaling by inflammatory monocytes is important for protection against DSS-induced colitis. Furthermore, both Il18−/− and Il18r1−/− monocytes isolated from DSS-treated mice have impaired production of TNFα similar to that observed with Nlrp6−/− monocytes, consistent with a role for NLPR6 in regulating TNFα production in monocytes via IL-18. Although IL-18 is typically associated with the upregulation of Th1 responses, and in particular, IFNγ,40 IL-18 has also been shown to activate monocytes to enhance TNFα production,41, 42 consistent with our data.
In summary, our studies highlight a previously unrecognized role for NLRP6 in modulating IL-18-dependent TNFα production in inflammatory Ly6Chi monocytes that can act as early responders to an inflammatory insult, thereby limiting bacterial translocation, promoting timely epithelial repair, and preventing the onset of dysregulated inflammation. Our data additionally suggest that early production of IL-18 to promote resistance against DSS-induced colitis is mediated not only by the intestinal epithelium, but also by inflammatory monocytes through NLRP6. Together, these results suggest that modulation of NLRP6 activity may be a potential strategy for the prevention and treatment of inflammatory bowel disease.
METHODS
Animal procedures. Adult 7–11 weeks old C57BL/6 WT, Nlrp6−/−, Il18−/− or Il18r1−/−, and GFP+ transgenic C57BL/6 (C57BL/6-Tg(UBC-GFP)30Scha/J) mice were bred in-house under specific pathogen-free conditions at the University of Michigan. WT, Il18−/− and Il18r1−/−,GFP+, CCR2−/−, and CX3CR1gfp mice (all B6 background) were originally purchased from Jackson (Bar Harbor, ME). Nlrp6−/− in B6 background were previously described.1 Animal studies were conducted under protocols approved by the University of Michigan Committee on the Use and Care of Animals.
DSS-induced colitis and AOM/DSS-induced tumorigenesis. To induce colitis and lethality, mice were treated with 3.5% DSS (MP Biomedicals; m.w. 36,000–50,000Da) in regular drinking water for 7 days. To develop colitis-associated tumors, mice were first injected with 10 mg kg−1 AOM(Sigma) i.p., followed by 5 days of 2% DSS starting on day 5. Mice were allowed to recover for 16 days with regular drinking water, and then treated with two additional cycles of 2% DSS (Figure 2a). Mice were sacrificed after the last cycle of DSS for tumor counting and size measurement by calipers.
Isolation of lamina propria cells. LP cells were isolated as previously described.1 Briefly, colons were cut into small pieces in HBSS buffer (Gibco) supplemented with 2.5% heat-inactivated FBS (Gibco, Gran Island, NY) (HBSS+), and washed with magnetic stirring at 37 °C. Colon pieces were then incubated in HBSS+/1 mM DTT at 37 °C followed by additional washes and incubation in HBSS+/1 mM EDTA. The supernatant containing IECs and IELs was saved for further analysis. The remaining colon pieces were further digested with 400 IU ml−1 Type III collagenase (Worthington) and 10 g ml−1 DNase I (Worthington) for two hours at 37 °C. When all tissue was digested, the cell suspension was filtered through a 70 μm filter, before running on a 75%/40% Percoll gradient to collect enriched LP cells from the interface.
Flow cytometry. Cell suspensions were treated with anti-CD16/CD32 (2.4G2) and then surface stained with combinations of the following fluorochrome-conjugated antibodies: from Biolegend (San Diego, CA): CD3e–FITC (clone: 145-2C11), Ly6C–FITC or APC/Cy7 (clone: HK1.4), Ly6G–PE/Cy7 (clone: 1A8), CD3e–APC/Cy7 (clone: 145-2C11), CD45R/B220–Pacific Blue (clone: RA3-6B2), CCR2-AlexaFluor647 (clone: SA203G11), CX3CR1-APC (clone: SA011F11), IgG2A,κ-APC isotype control; from BD Biosciences (San Jose, CA): NK1.1–PerCP/Cy5.5 (clone: PK136); from Ebiosciences (San Diego, CA): F4/80–APC (clone: BM8), CD4 (L3T4)–FITC (clone: RM4-5), CD11b–FITC (clone: M1/70), CD11b–PE (clone: M1/70), CD4–APC (clone: GK1.5). Cells were acquired on FACSCanto or AriaIII flow cytometers using FACSDiVa software, and data were analyzed using FlowJo software (Tree Star, Ashland, OR).
Adoptive transfer experiments. WT, Ccr2−/− or CX3CR1gfp mice were used as donors. Single-cell suspensions were prepared from flushed BM in fully supplemented RPMI followed by lysis of red blood cells and isolation of Ly6Chi monocytes (CD3-CD11b+Ly6ChiLy6G−) by FACs sorting. Two million cells were injected i.v. into Nlrp6−/−, WT or Ccr2−/− recipient mice.
Treatment of mice with recombinant TNFα. WT or Nlrp6−/− mice were administered 1 μg of sterilely filtered recombinant mouse TNFα (PreproTech) i.p. on days 3-7 in the 3.5% DSS colitis model (days 3–7) or days 8–10 in the AOM/DSS colitis and tumorigenesis model.
Assessment of colon inflammation. Colons were flushed free of feces, opened longitudinally, and jelly rolled for formalin fixation and paraffin embedding. Five-μm sections were used for H&E staining. Histological assessment was performed in a blinded fashion by a pathologist (KE) using a previously described scoring system1 with modifications. Entire colon sections were assessed for three main parameters: inflammatory cell infiltration, hyperplasia, and epithelial damage that were individually scored (0 to 4), (Supplementary Table 1). For each mouse, a weighted percent average based on the number of fields with a certain score was calculated for each parameter and then summed to obtain the final score. Assessment of colon weight after DSS treatment was performed by measuring the colon weight (excluding the cecum) after removal of feces normalized by its length (cm).
Intestinal permeability. Mice were fasted for 8 h with the exception of drinking water prior to the administration of 0.6 mg kg−1 FITC-dextran (4 kDa; Sigma-Aldrich, St. Louis, MO) by oral gavage. Serum was collected 4 h later, diluted 1:3 in phosphate-buffered saline (PBS), and fluorescence measured using a fluorescent spectrophotometer with emission at 488 nm and absorption at 525 nm.
Reverse transcription and qPCR. Total RNA was isolated from LP, BM cells, or from sorted populations of LP cells using the Nucleospin RNA kit (Machery-Nagel, Bethlehem, PA). cDNA synthesis was performed using iScript (Bio-Rad, Hercules, CA), and cDNA was used for quantitative PCR (qPCR) using the SYBR Green Master Mix (Applied Biosystems, Foster City, CA) on the ABI 7900HT (Applied Biosystems). Ct values were normalized to the β-actin gene. Primer sequences are available upon request.
ELISA. To measure fecal lipocalin-2 levels, fecal samples were homogenized in PBS at 100 mg ml−1 and supernatant was further diluted in a range of 1:500 to 1:5,000 for lipocalin-2/NGAL enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN). TNFα, IL-6 and IL-1β ELISAs (all R&D Systems) were performed with 24-hour culture supernatants of Ly6Chi monocytes.
Measurement of ROS. Total cellular ROS were measured by CellROX Deep Red reagent kit (Invitrogen) according to the manufacturer’s protocol. Briefly, upon surface staining for flow cytometry, cells were resuspended in 200 μl of pre-warmed HBSS+2.5 mM Probenecid+CellROX, incubated for 30 minutes at 37 °C (in the dark), washed and acquired on FACSCanto or AriaIII flow cytometers (BD Bioscience). CellROX Deep Red has peak excitation and emission at 640 and 665 nm.
Phagocytosis assay. eGFP-expressing E. coli (K12) was a kind gift from Gabriel Nunez (University of Michigan). Ly6Chi monocytes were specifically sorted from WT or Nlrp6−/− mice at day 10 of AOM/DSS and incubated for 1.5 h with E. coli-GFP at multiplicity of infection (MOI) 0, 5, 25, or 60. Zombie Violet viability die (Biolegend) was used to identify live Ly6Chi monocytes, which phagocytized GFP+ E. coli using FACSCanto or AriaIII flow cytometers (BD Bioscience).
Bacterial translocation. MLNs were homogenized in PBS by passing through a 70 μm strainer and then plated on Trypticase Soy Agar with 5% Sheep Blood (TSA II) plates (BD) to determine CFUs after 24-h incubation under aerobic conditions or 48-h under anaerobic conditions. Total DNA was extracted using the Nucleospin kit (Machery-Nagel), and bacterial load quantified by qPCR the universal 16S rRNA gene primers EUB-For-5′-AGAGTTTGATCCTGGCTC-3′ and EUB-Rev-5′-TGCTGCCTCCCGTAGGAGT-3′.
Statistical analysis. Statistically significant differences were determined using two-way analysis of variance (ANOVA) with a Bonferroni post hoc test (time × genotype, P<0.05), by one-way ANOVA with a Student-Newman-Keuls post hoc test (P value<0.05), or by two-tailed Student’s unpaired t-test when only two groups are compared. Survival curve comparison was performed by Mantel-Cox log-rank test. Kruskal–Wallis one-way analysis of variance non-parametric test was used for non-continuous variables. Data are shown as mean±s.e.m. GraphPad Prism6 software was utilized for statistical analysis.43, 44, 45
References
Chen, G.Y., Liu, M., Wang, F., Bertin, J. & Nunez, G. A functional role for Nlrp6 in intestinal inflammation and tumorigenesis. J. Immunol. 186, 7187–7194 (2011).
Chen, G.Y. Role of Nlrp6 and Nlrp12 in the maintenance of intestinal homeostasis. Eur. J. Immunol. 44, 321–327 (2013).
Elinav, E. et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 145, 745–757 (2011).
Normand, S. et al. Nod-like receptor pyrin domain-containing protein 6 (NLRP6) controls epithelial self-renewal and colorectal carcinogenesis upon injury. Proc. Natl. Acad. Sci. USA 108, 9601–9606 (2011).
Allen, I.C. et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J. Exp. Med. 207, 1045–1056 (2010).
Zaki, M.H., Boyd, K.L., Vogel, P., Kastan, M.B., Lamkanfi, M. & Kanneganti, T.D. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 32, 379–391 (2010).
Wlodarska, M. et al. NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell 156, 1045–1059 (2014).
Gordon, S. & Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964 (2005).
Zigmond, E. et al. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity 37, 1076–1090 (2012).
Sherwood, R.A. Faecal markers of gastrointestinal inflammation. J. Clin. Pathol. 65, 981–985 (2012).
Murray, P.J. & Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 11, 723–737 (2011).
Anand, P.K. et al. NLRP6 negatively regulates innate immunity and host defence against bacterial pathogens. Nature 488, 389–393 (2012).
Kempster, S.L. et al. Developmental control of the Nlrp6 inflammasome and a substrate, IL-18, in mammalian intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G253–G263 (2010).
Reinecker, H.C. et al. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin. Exp. Immunol. 94, 174–181 (1993).
Rakoff-Nahoum, S., Paglino, J., Eslami-Varzaneh, F., Edberg, S. & Medzhitov, R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118, 229–241 (2004).
Pizarro, T.T. et al. IL-18, a novel immunoregulatory cytokine, is up-regulated in Crohn's disease: expression and localization in intestinal mucosal cells. J. Immunol. 162, 6829–6835 (1999).
Furuya, D. et al. Serum interleukin-18 concentrations in patients with inflammatory bowel disease. J. Immunother. 25, S65–S67 (2002).
Salcedo, R. et al. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. J. Exp. Med. 207, 1625–1636 (2010).
Siegmund, B. Interleukin-18 in intestinal inflammation: friend and foe? Immunity 32, 300–302 (2010).
Dupaul-Chicoine, J. et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity 32, 367–378 (2010).
Huber, S. et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature 491, 259–263 (2012).
Naito, Y. et al. Enhanced intestinal inflammation induced by dextran sulfate sodium in tumor necrosis factor-alpha deficient mice. J. Gastroenterol. Hepatol. 18, 560–569 (2003).
Noti, M., Corazza, N., Mueller, C., Berger, B. & Brunner, T. TNF suppresses acute intestinal inflammation by inducing local glucocorticoid synthesis. J. Exp. Med. 207, 1057–1066 (2010).
Wang, Y. et al. Protective role of tumor necrosis factor (TNF) receptors in chronic intestinal inflammation: TNFR1 ablation boosts systemic inflammatory response. Lab. Invest. 93, 1024–1035 (2013).
Zhan, Y. et al. Gut microbiota protects against gastrointestinal tumorigenesis caused by epithelial injury. Cancer Res. 73, 7199–7210 (2013).
Edelblum, K.L., Goettel, J.A., Koyama, T., McElroy, S.J., Yan, F. & Polk, D.B. TNFR1 promotes tumor necrosis factor-mediated mouse colon epithelial cell survival through RAF activation of NF-kappaB. J. Biol. Chem. 283, 29485–29494 (2008).
Feng, Y. & Teitelbaum, D.H. Tumour necrosis factor—induced loss of intestinal barrier function requires TNFR1 and TNFR2 signalling in a mouse model of total parenteral nutrition. J. Physiol. 591, 3709–3723 (2013).
Stillie, R. & Stadnyk, A.W. Role of TNF receptors, TNFR1 and TNFR2, in dextran sodium sulfate-induced colitis. Inflamm. Bowel Dis. 15, 1515–1525 (2009).
Wang, K. et al. Opposite role of tumor necrosis factor receptors in dextran sulfate sodium-induced colitis in mice. PLoS One 7, e52924 (2012).
Naude, P.J., den Boer, J.A., Luiten, P.G. & Eisel, U.L. Tumor necrosis factor receptor cross-talk. FEBS J. 278, 888–898 (2011).
Hijdra, D., Vorselaars, A.D., Grutters, J.C., Claessen, A.M. & Rijkers, G.T. Differential expression of TNFR1 (CD120a) and TNFR2 (CD120b) on subpopulations of human monocytes. J. Inflamm. (Lond) 9, 38 (2012).
Greten, F.R. et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118, 285–296 (2004).
Bain, C.C. & Mowat, A.M. The monocyte-macrophage axis in the intestine. Cell Immunol. 291, 41–48 (2014).
Bain, C.C. & Mowat, A.M. Macrophages in intestinal homeostasis and inflammation. Immunol. Rev. 260, 102–117 (2014).
Bain, C.C. et al. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 6, 498–510 (2013).
Varol, C., Zigmond, E. & Jung, S. Securing the immune tightrope: mononuclear phagocytes in the intestinal lamina propria. Nat. Rev. Immunol. 10, 415–426 (2010).
Qualls, J.E., Kaplan, A.M., van Rooijen, N. & Cohen, D.A. Suppression of experimental colitis by intestinal mononuclear phagocytes. J. Leukoc. Biol. 80, 802–815 (2006).
Molloy, M.J. et al. Intraluminal containment of commensal outgrowth in the gut during infection-induced dysbiosis. Cell Host Microbe 14, 318–328 (2013).
Bayir, H. Reactive oxygen species. Crit. Care Med. 33, S498–S501 (2005).
Nakanishi, K., Yoshimoto, T., Tsutsui, H. & Okamura, H. Interleukin-18 regulates both Th1 and Th2 responses. Annu. Rev. Immunol. 19, 423–474 (2001).
Dai, S.M., Matsuno, H., Nakamura, H., Nishioka, K. & Yudoh, K. Interleukin-18 enhances monocyte tumor necrosis factor alpha and interleukin-1beta production induced by direct contact with T lymphocytes: implications in rheumatoid arthritis. Arthritis Rheum. 50, 432–443 (2004).
Dias-Melicio, L.A., Fernandes, R.K., Rodrigues, D.R., Golim, M.A. & Soares, A.M. Interleukin-18 increases TLR4 and mannose receptor expression and modulates cytokine production in human monocytes. Mediators Inflamm. 2015, 236839 (2015).
Ahmadian, M. et al. PPARgamma signaling and metabolism: the good, the bad and the future. Nat. Med. 19, 557–566 (2013).
Adachi, M. et al. Peroxisome proliferator activated receptor gamma in colonic epithelial cells protects against experimental inflammatory bowel disease. Gut 55, 1104–1113 (2006).
Shah, Y.M., Morimura, K. & Gonzalez, F.J. Expression of peroxisome proliferator-activated receptor-gamma in macrophage suppresses experimentally induced colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G657–G666 (2007).
Acknowledgements
This work was supported by the National Institutes of Health grant R01CA166879 and American Cancer Society Research Scholar Grant awarded to GYC, National Institutes of Health grant F32CA200144 awarded to SSS and by grant number UL1TR000433 from the National Center for Advancing Translational Sciences awarded to SSS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Supplementary information
Rights and permissions
About this article
Cite this article
Seregin, S., Golovchenko, N., Schaf, B. et al. NLRP6 function in inflammatory monocytes reduces susceptibility to chemically induced intestinal injury. Mucosal Immunol 10, 434–445 (2017). https://doi.org/10.1038/mi.2016.55
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2016.55
This article is cited by
-
Inhibition of CD82 improves colitis by increasing NLRP3 deubiquitination by BRCC3
Cellular & Molecular Immunology (2023)
-
Epithelial Nlrp10 inflammasome mediates protection against intestinal autoinflammation
Nature Immunology (2023)
-
Physiological and pathophysiological functions of NLRP6: pro- and anti-inflammatory roles
Communications Biology (2022)
-
LncRNA H19 initiates microglial pyroptosis and neuronal death in retinal ischemia/reperfusion injury
Cell Death & Differentiation (2020)
-
1,25(OH)2D3 deficiency-induced gut microbial dysbiosis degrades the colonic mucus barrier in Cyp27b1 knockout mouse model
Gut Pathogens (2019)