Abstract
Objective:
To examine the effect of sildenafil therapy on development of severe retinopathy of prematurity (ROP) requiring surgical intervention in premature infants.
Study Design:
We identified premature infants who were discharged from Pediatrix Medical Group neonatal intensive care units from 2003 to 2012 and who received an ophthalmologic exam. We matched each infant exposed to sildenafil before first eye exam to three nonexposed infants using propensity scoring to control for differences in baseline infant characteristics. We evaluated the association between sildenafil exposure and development of severe ROP using conditional logistic regression.
Result:
Of the 57 815 infants meeting inclusion criteria, 88 were exposed to sildenafil. We matched 81/88 (92%) sildenafil-exposed with 243 nonexposed infants. There was no difference in the proportion of infants who developed severe ROP in the sildenafil-exposed vs nonexposed groups (17/81 (21%) vs 38/243 (16%), P=0.27). On adjusted analysis, there was no difference in severe ROP in the sildenafil-exposed vs nonexposed infants (odds ratio=1.46, 95% confidence interval=0.76 to 2.82, P=0.26).
Conclusion:
We did not observe an association between risk of severe ROP and sildenafil exposure before first eye exam in this cohort of premature infants.
Similar content being viewed by others
Introduction
Sildenafil is increasingly used off-label for treatment of pulmonary hypertension (PH) of various etiologies in infants and children.1 Persistent pulmonary hypertension of the newborn and PH associated with congenital heart disease, chronic lung disease or congenital diaphragmatic hernia are common conditions treated with sildenafil therapy in term and preterm infants.2
The therapeutic effect of sildenafil in PH occurs through its inhibitory action on phosphodiesterase type 5 (PDE5) that results in nitric oxide-mediated vasorelaxation.3 Despite the high selectivity of sildenafil for PDE5 enzymes, it also inhibits the retina-specific phosphodiesterase type 6 (PDE6) with one-tenth the potency compared with PDE5.3 Expression of PDE6 enzymes in rod and cone photoreceptors of retinal tissue, as well as the discovery of PDE5 enzymes on retinal and choroid vasculature, have raised concerns about potential adverse effects of sildenafil on the developing eye of premature infants.3, 4, 5
Because of the increasing use of sildenafil despite the lack of population-specific safety data,6 we sought to examine the association between sildenafil therapy and the development of severe retinopathy of prematurity (ROP), requiring therapy in hospitalized, very low birth weight infants (≤1500 g).
Methods
Data source
Data were obtained from the Pediatrix Medical Group Data Warehouse that prospectively captures information from an electronic medical record of daily progress notes and other documentation of clinicians involved in the care of infants (https://www.pediatrix.com/PediatrixUniversity). We included all inborn infants ≤32 weeks of gestational age (GA) and ≤1500 g birth weight who received an ophthalmologic exam and were discharged from one of the 326 North American neonatal intensive care units managed by the Pediatrix Medical Group from 2003 to 2012. Infants who started sildenafil therapy after their initial ROP examination or after 36 weeks of postmenstrual age were excluded. Information regarding maternal history, infant demographics, respiratory and hemodynamic support, medications, culture results and ophthalmologic exams were obtained.
Definitions
The primary outcome of our study was severe ROP, defined as any ROP requiring surgical intervention, cryotherapy, laser therapy or treatment with bevacizumab. We defined sildenafil exposure as any sildenafil therapy before the initial ophthalmologic examination, and calculated cumulative exposure to sildenafil as the number of days of exposure to sildenafil before the initial ophthalmologic examination. We defined history of bacteremia as a binary variable if the infant had at least one positive blood culture with organisms not considered a contaminant before or on the infant day of hospitalization. We defined cumulative daily inotropic support, mechanical ventilation and supplemental oxygen as the cumulative days of exposure to any inotropic drug (dobutamine, dopamine, epinephrine, milrinone/amrinone, norepinephrine), any mechanical ventilation and any fraction of inspired oxygen (FiO2) >21% up until each infant day of hospitalization. We defined small for gestational age as previously described.7
Statistical analysis
We used medians with interquartile ranges and counts with percentages to describe continuous and categorical variables, respectively. We compared the distribution between infants with and without sildenafil exposure using Wilcoxon rank-sum, χ2 and Fisher’s exact tests where appropriate. Because more severely ill infants are more likely to receive sildenafil therapy and are at higher risk of developing ROP, we used propensity scores to match infants in a 3:1 ratio (nonexposed/exposed) to ensure comparison of similar infants.8 A propensity score for sildenafil exposure or nonexposure on each infant day of hospitalization was estimated using multivariable logistic regression including covariates that might predict both sildenafil therapy and ROP risk: GA in weeks, postnatal age in days, sex, race/ethnicity, Apgar score at 5 min, small for gestational age status, antenatal steroid exposure, bacteremia, mechanical ventilation, inotropic support and supplemental oxygen, as defined above.9 We matched infants on the first day of sildenafil exposure with three nonexposed infants using nearest-neighbor matching without replacement. Matching was allowed only if the difference in propensity score between case and control was <0.01. We assessed covariate balance across treatment groups by comparing covariate means, histograms and Kernel density plots of propensity scores across the two groups. We used Stata’s psmatch2 routine that implements full Malhanobis and a variety of propensity score matching methods.10 We compared the distribution of covariates between the two groups after propensity matching using Wilcoxon rank-sum, χ2 or Fisher’s exact tests where appropriate. We used conditional (fixed effects) logistic regression to evaluate the association between any sildenafil exposure before ROP evaluation and risk of severe ROP conditioned on the matched pair. In an a priori defined secondary analysis, we used conditional logistic regression to evaluate the association between risk of severe ROP and cumulative duration of sildenafil exposure as defined above, and categorized as 0 days, 1 to 7 days, 8 to 14 days and >14 days. The study was approved by the Duke University institutional review board without the need for written informed consent as the data sets used did not contain any patient identifiers.
Results
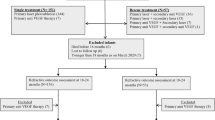
We identified 57 815 very low birth weight infants meeting inclusion criteria, of whom 88 (0.2%) were exposed to sildenafil (Figure 1). The median (25 to 75th percentile) birth weight and GA of the entire cohort were 1006 g (830 to 1270) and 28 weeks (26 to 30), respectively. Median GA and birth weight were lower in infants exposed to sildenafil compared with those not exposed (GA 25 weeks (24 to 27) vs 28 weeks (26 to 30), P<0.001, and birth weight 665 g (535 to 813) vs 1006 g (833 to 1270), P<0.001). Infants exposed to sildenafil were also more likely to be small for gestational age, had lower 5 min Apgar scores and more exposure to inotropic drugs, ventilator support and supplemental oxygen compared with those not exposed to sildenafil (Table 1). The proportion of infants who developed severe ROP was higher in the sildenafil-exposed compared with the nonexposed group (18/88 (21%) vs 2546/57 727 (4%), P<0.001).
We successfully matched 81/88 (92%) sildenafil-exposed with 243/57 727 (<1%) nonexposed infants. Following matching, characteristics between groups were well balanced, and there were no statistically significant differences between the sildenafil-exposed and nonexposed infants in baseline demographics, cumulative days of inotropic exposure, mechanical ventilation or oxygen therapy. Median postnatal age at the time of severe ROP therapy also did not differ significantly between sildenafil-exposed and nonexposed infants (93 days (74 to 99) vs 87 days (73 to –102), P=0.95), nor did median PMA (37 weeks (36 to 38) vs 37 weeks (35 to 39), P=0.86).
After matching, the proportion of infants who developed severe ROP was similar in the sildenafil-exposed vs nonexposed infants (17/81 (21%) vs 38/243 (16%), P=0.27). There was no significant difference in the odds of severe ROP in the sildenafil-exposed vs nonexposed infants (odds ratio=1.46, 95% confidence interval=0.76 to 2.82, P=0.26). The median duration of sildenafil therapy was 10 days (2–31). Duration of sildenafil therapy was 1 to 7 days in 35/81 infants (43%), 8 to 14 days in 10/81 infants (12%) and >14 days in 36/81 infants (44%). Increasing duration of sildenafil exposure before initial eye exam was also not associated with increased risk for development of severe ROP compared with the nonexposed infants (Table 2).
Discussion
We present the results of a propensity score-matched, case–control study of hospitalized very low birth weight infants exposed to sildenafil before their first ROP evaluation. Following matching, groups were well balanced for available risk factors of ROP, and we observed no difference in the risk of severe ROP in infants exposed to sildenafil. This observation remained when using duration of sildenafil exposure as a predictor of severe ROP.
Reported potential ocular adverse effects of sildenafil in infants are limited. A case report described a 26-week premature infant who developed aggressive ROP while receiving sildenafil therapy.5 The infant had a complicated clinical course, including three episodes of bacterial and fungal sepsis, and required 100% oxygen therapy for up to 32 weeks corrected GA. In a retrospective, single-center, case–control study, 17 infants <30 weeks of gestation exposed to sildenafil were compared with 51 control infants for progression of ROP or need for laser treatment.11 Sildenafil was started at median of 36 weeks corrected GA and continued for a median duration of 52 days. The treated and untreated groups were not similar in their baseline characteristics, with infants in the sildenafil group requiring more respiratory support. The time of ROP assessment relative to the timing of sildenafil therapy was unclear. This study did not observe any effect of sildenafil on ROP progression or need for laser treatment. Another retrospective, single-center study assessed 22 term and later preterm infants not at risk for ROP or for development of any other ocular complications following 2 to 36 weeks of treatment with sildenafil.12 All infants received an ophthalmologic assessment at chronological age of 2 to 40 weeks indicated specifically because of sildenafil therapy. This study did not find any ocular complications related to the use of sildenafil.
We report the first multicenter study evaluating the association between early sildenafil exposure and severe ROP. In addition to larger sample size, we were able to control for several known ROP risk factors in our analysis through propensity score matching. This matching was essential given the significant differences in ROP risk factors between the unmatched populations. Our results are in agreement with the existing limited evidence that sildenafil therapy does not increase the risk for development of severe ROP in premature infants.
Sildenafil is a predominant PDE5 inhibitor with vasodilatory properties in widespread use since its initial approval in adults.13 The promising results of sildenafil for treatment of PH in adults have resulted in its increasing use in infants.1, 2 In a recent review of medication use in hospitalized infants, sildenafil had the second highest relative increase in use, up by over 11-fold, from 0.2 to 2.3 per 1000 infants from 2005 to 2010.6 Reports of visual disturbances in adults treated for erectile dysfunction, although mostly mild and transient, drew considerable attention to the potential adverse effects of sildenafil on the ocular system.14, 15, 16, 17, 18 These effects have been attributed to its cross-inhibition of the retinal photoreceptor-specific PDE6 enzyme, as well as the recent discovery of PDE5 enzymes in the retinal and choroidal vasculature.19 Alterations of the nitric oxide signaling pathway, increase in intraocular pressure, changes in choroidal blood flow or thickness and variation of retinal perfusion have been raised as potential mechanisms for the observed adverse effect of sildenafil on the ocular system.20, 21, 22 Nitric oxide is an important determinant of choroidal and retinal blood flow in the developing eye of premature infants. It is known to have inhibitive effect on retinal vascular obliteration and subsequently proliferative retinopathies, but it can also increase neoangiogenic activity via vascular endothelial growth factor-induced angiogenesis.23 It is not known whether any of these sildenafil-induced changes in nitric oxide signaling pathways affects the development and progression of ROP.
Our study is limited in its retrospective nature and data obtained from electronic documentation of clinical care rather than from a prospectively established research database. Most importantly, at the time of this analysis, sildenafil dosing and indication were not completely captured in the database, and we were unable to examine any potential associations between sildenafil dosing, exposure and ROP risk. This limitation is particularly relevant as the ocular safety of sildenafil may be dose dependent. Dose dependency has been suggested in rat models of PDE-6 expression that is increasingly downregulated with higher doses of sildenafil.24, 25 Moreover, details from the ocular examination, including exact timing of diagnosis or early findings suggestive of increased ROP risk and severity and extent of ROP, were not available, and we were unable to stratify infants according to the International Classification of Retinopathy of Prematurity system.26 Instead, we had to use ROP intervention during hospitalization as a surrogate for severity, and the decision to treat ROP was at the discretion of the local provider. Outpatient follow-up data to identify later diagnoses of ROP or resulting blindness were also not available. We examined infants with early sildenafil exposure and used propensity score matching, resulting in balanced groups with regard to baseline risk of ROP.27 Our results add to the safety profile of sildenafil as an off-label medication in infants and show that infants who were exposed to sildenafil at a very young age did not have a higher risk for development of severe ROP.
Conclusion
We did not observe an increased risk of ROP in premature infants treated with sildenafil before their first ROP exam. These results support the conduct of future prospective trials characterizing the safety and efficacy of sildenafil in premature infants.
References
Malik M, Nagpal R . Emerging role of sildenafil in neonatology. Indian Pediatr 2011; 48 (1): 11–13.
Samiee-Zafarghandy S, Smith PB, van den Anker J . Safety of sildenafil in infants. Pediatr Crit Care Med 2014; 15 (4): 362–368.
Cordell W . Retinal effects of 6 months of daily use of tadalafil or sildenafil. Arch Ophthalmol 2009; 127 (4): 367.
Azzouni F, Abu Samra K . Are phosphodiesterase type 5 inhibitors associated with vision-threatening adverse events? A critical analysis and review of the literature. J Sex Med 2011; 8 (10): 2894–2903.
Marsh C . Severe retinopathy of prematurity (ROP) in a premature baby treated with sildenafil acetate (Viagra) for pulmonary hypertension. Br J Ophthalmol 2004; 88 (2): 306–307.
Hsieh E, Hornik C, Clark R, Laughon M, Benjamin D, Smith PB . Medication use in the neonatal intensive care unit. Am J Perinatol 2013; 31 (9): 811–822.
Olsen I, Groveman S, Lawson M, Clark R, Zemel B . New intrauterine growth curves based on United States data. Pediatrics 2010; 125 (2): e214–e224.
Rubin DB, Thomas N . Matching using estimated propensity scores: relating theory to practice. Biometrics 1996; 52 (1): 249–264.
Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Sturmer T . Variable selection for propensity score models. Am J Epidemiol 2006; 163 (12): 1149–1156.
Leuven E, Sianesi B . Psmatch2: stata module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing. Available from http://ideas.repec.org/c/boc/bocode/s432001.html Created 2003; revised 2015.
Fang A, Guy K, König K . The effect of sildenafil on retinopathy of prematurity in very preterm infants. J Perinatol 2012; 33 (3): 218–221.
Kehat R, Bonsall D, North R, Connors B . Ocular findings of oral sildenafil use in term and near-term neonates. J AAPOS 2010; 14 (2): 159–162.
Laties A . Vision disorders and phosphodiesterase type 5 inhibitors. Drug Saf 2009; 32 (1): 1–18.
Center for Drug Evaluation and Research NDA 020895/S-21 Viagra (Sildenafil Citrate) Tablets: Clinical Pharmacology/Biopharmaceutics Review. Department of Health and Human Services, US Food and Drug Administration: Rockville, MD, 1998.
Center for Drug Evaluation and Research Study 148–223: a double-blind, randomized, placebo-controlled, four-period crossover study to assess the effect of orally administered sildenafil (50, 100, and 200 mg) on visual function in healthy male volunteers. In: Viagra (Sildenafil): Joint Clinical Review for NDA-20-895. Center for Drug Evaluation and Research, FDA: Washington, DC, 1998, pp 160–161.
Donahue S, Taylor R . Pupil-sparing third nerve palsy associated with sildenafil citrate (Viagra). Am J Ophthalmol 1998; 126 (3): 476–477.
Egan R, Pomeranz H . Sildenafil (Viagra) associated anterior ischemic optic neuropathy. Arch Ophthalmol 2000; 118 (2): 291–292.
Cunningham A, Smith K . Anterior ischemic optic neuropathy associated with Viagra. J Neuroophthalmol 2001; 21 (1): 22–25.
Foresta C, Caretta N, Zuccarello D, Poletti A, Biagioli A, Caretti L et al. Expression of the PDE5 enzyme on human retinal tissue: new aspects of PDE5 inhibitors ocular side effects. Eye 2007; 22 (1): 144–149.
Gerometta R, Alvarez L, Candia O . Effect of sildenafil citrate on intraocular pressure and blood pressure in human volunteers. Exp Eye Res 2011; 93 (1): 103–107.
Harris A, Kagemann L, Ehrlich R, Ehrlich Y, Lopez C, Purvin V . The effect of sildenafil on ocular blood flow. Br J Ophthalmol 2008; 92 (4): 469–473.
Vance S, Imamura Y, Freund K . The effects of sildenafil citrate on choroidal thickness as determined by enhanced depth imaging optical coherence tomography. Retina 2011; 31 (2): 332–335.
Cavallaro G, Filippi L, Bagnoli P, La Marca G, Cristofori G, Raffaeli G et al. The pathophysiology of retinopathy of prematurity: an update of previous and recent knowledge. Acta Ophthalmol 2013; 92 (1): 2–20.
Heywood R, Osterloh IH, Phillips SC . Sildenafil causes a dose- and time-dependent downregulation of phosphodiesterase type 6 expression in the rat retina. Int J Impot Res 2000; 12 (4): 241–244.
Jackson G, Benjamin N, Jackson N, Allen MJ . Effects of sildenafil citrate on human hemodynamics. Am J Cardiol 1999; 83 (5A): 13C–20C.
International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol 2005; 123 (7): 991–999.
Misra A, Heckford E, Curley A, Allen L . Do current retinopathy of prematurity screening guidelines miss the early development of pre-threshold type 1 ROP in small for gestational age neonates? Eye 2007; 22 (6): 825–829.
Acknowledgements
This work was performed under the Best Pharmaceuticals for Children Act – Pediatric Trials Network (Government Contract HHSN275201000003I).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
Dr Smith receives salary support for research from the National Institutes of Health (NIH) and the National Center for Advancing Translational Sciences of the NIH (UL1TR001117), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (HHSN275201000003I and 1R01-HD081044-01) and the Food and Drug Administration (1R18-FD005292-01); he also receives research support from Cempra Pharmaceuticals (subaward to HHS0100201300009C) and industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp). Dr van den Anker receives salary support for research from the NIH (5K24DA027992, 5U54HD071601, 5R01HD060543). Dr Hornik receives salary support for research from the National Center for Advancing Translational Sciences of the NIH (UL1TR001117). Dr Laughon receives support from the US government for his work in pediatric and neonatal clinical pharmacology (HHSN267200700051C, PI: Benjamin, under the Best Pharmaceuticals for Children Act) and from the NICHD (5K23HD068497-01). The other authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Journal of Perinatology website
Supplementary information
Rights and permissions
About this article
Cite this article
Samiee-Zafarghandy, S., van den Anker, J., Laughon, M. et al. Sildenafil and retinopathy of prematurity risk in very low birth weight infants. J Perinatol 36, 137–140 (2016). https://doi.org/10.1038/jp.2015.126
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2015.126
This article is cited by
-
Early diagnosis and targeted approaches to pulmonary vascular disease in bronchopulmonary dysplasia
Pediatric Research (2022)
-
Prophylactic Sildenafil in Preterm Infants at Risk of Bronchopulmonary Dysplasia: A Pilot Randomized, Double-Blinded, Placebo-Controlled Trial
Clinical Drug Investigation (2019)
-
Controversies in the identification and management of acute pulmonary hypertension in preterm neonates
Pediatric Research (2017)
-
More safety data: what about efficacy of sildenafil?
Journal of Perinatology (2016)