Abstract
Werner mesomelic syndrome (WMS), an autosomal dominant disorder characterized by hypoplastic tibiae, triphalangeal thumbs and polydactyly, is caused by a specific point mutation at the position 404 in zone of polarizing activity regulatory sequence (ZRS). Here we identified two additional families with WMS. All three patients in three generations of Family 1 were found to harbor the same heterozygous 406A>G mutation in ZRS. The fourth patient from Family 2 was a sporadic case with the known 404 point mutation. The novel 406A>G mutation expands mutational spectrum in ZRS causing WMS, provides evidence for a functionally important nucleotide position 406 of ZRS in humans and has implications for genetic counseling.
Similar content being viewed by others
Introduction
Limb development requires the spatial and temporal organization of embryos, which is achieved through the spatial and temporal regulation of gene expression.1 This is controlled by transcription levels of multiprotein complexes and gene regulatory regions, including enhancers. Zone of polarizing activity (ZPA) regulatory sequence (ZRS), located within the intron 5 of LMBR1, acts as an enhancer regulating the expression of the Sonic Hedgehog (SHH) gene located ∼1 Mb downstream.2 SHH is secreted from the ZPA region of the posterior mesenchyme, along the anterior–posterior axis in the developing limb bud in a concentration gradient.3 ZRS is highly conserved from fish to mammals.4
Mutations in ZRS cause a wide spectrum of limb anomalies, collectively called ZRS-associated syndromes, ranging from preaxial polydactyly type II to Werner mesomelic syndrome (WMS; OMIM 188770).5 WMS is the most severe end of the phenotypic spectrum characterized by hypoplastic tibiae, triphalangeal thumbs and polydactyly. Interestingly, there is a certain genotype–phenotype correlation.6 Notably, all 15 reported patients with WMS from four unrelated families were heterozygous for a specific point mutation at position 404 of ZRS.6, 7, 8 Here we report two families with WMS. Three patients were found to harbor a novel 406A>G mutation in ZRS, remarkably expanding the mutational spectrum of WMS.
Materials and methods
Subjects
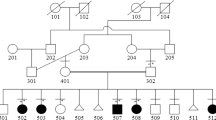
We identified four patients from two families with WMS. Their clinical and radiographic features were summarized in Table 1 and Figure 1. Family 1 had three affected members in three generations, while the fourth patient from Family 2 was sporadic (Figures 2a and b). After informed consent, their whole blood was obtained for mutation analysis.
Clinical and radiographic features of patients with WMS. (a and b) Hand photographs and radiographs, respectively, of patients F1:I-1, F1:II-1, F1:III-1 and F2:II-1. All had triphalangeal first fingers bilaterally. Patient F2:II-1 also had a preaxial extra digit on her right hand. (c and d) Lower-limb photographs and radiographs, respectively. (e and f) Feet photographs and radiographs, respectively. A full color version of this figure is available at the Journal of Human Genetics journal online.
(a) Pedigree of Family 1 showing three patients in three generations. (b) Pedigree of Family 2 showing our fourth patient as a sporadic case. (c) Chromatograms showing the 406A>G (middle panel) and 404G>A (lower panel) mutations comparing with wild-type (upper panel) sequence. The arrow heads and the arrows indicate the nucleotides at positions 404 and 406, respectively. (d) Sequence alignment showing the highly conserved region covering the altered 404G and 406A positions from fish to mammals. A full color version of this figure is available at the Journal of Human Genetics journal online.
Mutation analysis
DNA was extracted from leukocytes using QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instruction. PCR amplifications were carried out for the 772-bp ZRS region using 5′-CTGGCCAGTGTTTAAATGGT-3′ (forward) and 5′-TGATCCATAACCATTTCTAAG-3′ (reverse) primers,6 and Taq DNA polymerase (Fermentas, Glen Burnie, MD, USA). PCR products were treated with ExoSAP-IT (USP Corporation, Cleveland, OH, USA), and sent for direct sequencing at Macrogen (Seoul, Korea).
To confirm the variants identified in the patients and to determine their frequencies in unaffected family members and controls, pyrosequencing was carried out using the 5′-TTGTCCTGGTTTATGTCCCT-3′ (forward) and 5′-ATGAAAGCTCGTGGAGACAG-3′ (biotin-tagged reverse) primers and 5′-CATAAAAGTGACCTTGTACT-3′ sequencing primer.
Alignment data form Genome browser (http://genome.ucsc.edu/cgi-bin/hgGateway) were used to determine evolutionary conservation of the mutated nucleotides.
Results
Mutation analysis
PCR-Sanger sequencing of the ZRS region of all three affected members from Family 1 showed that they were heterozygous for the 406A>G mutation (Figure 2c). The sporadic patient from Family 2 was found to harbor a heterozygous 404G>A mutation (Figure 2c). These two mutations were absent in 200 alleles of healthy Thai individuals determined by pyrosequencing. The 404G and the 406A are evolutionarily conserved from fish to humans (Figure 2d).
Discussion
We reported clinical, radiographic and molecular features of four Thai patients with WMS. They showed both intra- and interfamilial variability. Tibial defects ranged from unilaterally normal, as in Patients F1:I-1 and F2:II-1, to bilaterally aplastic, as in Patients F1:II-1 and F1:III-1 (Figure 1). Nonetheless, one striking consistent feature in all four patients is bilateral triphalangeal first fingers. Instead of using two previously used nomenclatures,8, 9 triphalangeal thumbs6, 10, 11 or five-fingered hands,8, 12, 13 we here call the digits ‘triphalangeal first fingers’. This is to indicate that the digits have features of fingers, not thumbs (no thenar muscles, no first web spaces and inability to oppose) with three phalangeal bones but are at the position of the normal thumbs. The term ‘five-fingered hands’ could not be used in our fourth sporadic patient who had six digits on her right hand. Her preaxial extra digit should be called as it is not the first digit. If it is surgically removed, the digit at the position of the normal thumb can still be called the first finger.
In our Family 1 (Figure 2a), we observed not only clinical variability but also an increase in severity in the three generations. The grandfather (I-1) had normal left and hypoplastic right tibiae, while the father (II-1) had no tibiae bilaterally and the son (III-1) had no tibiae and dislocated knees bilaterally (Figure 1 and Table 1). Clinical variability and anticipation have previously been reported in WMS,7, 11 with no molecular explanation.
As the causative gene of WMS was identified in 2003, 15 patients from four families had undergone mutation analysis. They all harbored a heterozygous mutation at position 404 of ZRS.6, 7, 8 The six affected of the Cuban family,7 two affected of the Turkish family6 and one sporadic Korean case8 had the 404G>A mutation, whereas the six affected of the Brazilian family had the 404G>C mutation.6 Surprisingly, all three patients in Family 1 in our study were found to be heterozygous for a novel point mutation, 406A>G.
ZRS point mutations were found to change the balance of two groups of E-twenty-six (ETS) transcription factor binding sites14 or create binding sites for HnRNP U.15 These lead to ectopic Shh expression in limb buds,16 causing limb abnormalities. Although the ∼800-bp ZRS is highly conserved from fish to humans, many nucleotide positions in this region are different among species.17 In addition, the mutations lying in ZRS causing limb defects in different species vary.16 The heterozygous 406A>G was previously identified in the mouse M100081, which exhibited a tibial defect, preaxial polydactyly and triphangeal first digits,17 similar to those found in our patients. In fact, transgenic embryos carrying the 404 G>A and the 406 A>G mutations in the mouse ZRS showed similar expression pattern of Shh in the ZPA.16 Shh was ectopically expressed at the anterior margin of the limb bud, in addition to the normal posterior expression.16, 18 Our findings of the 406 mutation leading to WMS in humans, similar to the 404 mutation, not only identify the functional nucleotide, but also support the assumption that there are small sub-domains within the ZRS expected to delineate functional regulatory units in humans.16
Although the 406A>G mutation in our Family 1 was inherited, the origin of the 404G>A mutation in our Family 2 could not be determined because of the unavailability of the proband’s parental DNA. Nonetheless, it is possible that the 404G>A mutation in the proband of Family 2 is de novo, as previously shown in a sporadic case of WMS.8 As fetal genomic sequencing as a prenatal screening/diagnosis for healthy couples is on the horizon,19 identification of this new mutation leading to such severe phenotype would have an implication for genetic counseling.
Our newly identified 406 point mutation causing WMS in three patients not only expands mutational spectrum of ZRS causing WMS, but also demonstrates that the nucleotide 406 initially identified through mouse models is also critical for limb development in humans.
References
Towers, M. & Tickle, C. Growing models of vertebrate limb development. Development 136, 179–190 (2009).
Lettice, L. A., Horikoshi, T., Heaney, S. J., van Baren, M. J., van der Linde, H. C., Breedveld, G. J. et al. Disruption of a long-range cis-acting regulator for Shh causes preaxial polydactyly. Proc. Natl Acad. Sci. USA 99, 7548–7553 (2002).
Zeller, R., Lopez-Rios, J. & Zuniga, A. Vertebrate limb bud development: moving towards integrative analysis of organogenesis. Nat. Rev. Genet. 10, 845–858 (2009).
Dahn, R. D., Davis, M. C., Pappano, W. N. & Shubin, N. H. Sonic hedgehog function in chondrichthyan fins and the evolution of appendage patterning. Nature 445, 311–314 (2007).
Anderson, E., Peluso, S., Lettice, L. A. & Hill, R. E. Human limb abnormalities caused by disruption of hedgehog signaling. Trends Genet. 28, 364–373 (2012).
Wieczorek, D., Pawlik, B., Li, Y., Akarsu, N. A., Caliebe, A., May, K. J. et al. A specific mutation in the distant sonic hedgehog (SHH) cis-regulator (ZRS) causes Werner mesomelic syndrome (WMS) while complete ZRS duplications underlie Haas type polysyndactyly and preaxial polydactyly (PPD) with or without triphalangeal thumb. Hum. Mutat. 31, 81–89 (2010).
Lettice, L. A., Heaney, S. J., Purdie, L. A., Li, L., de Beer, P., Oostra, B. A. et al. A long-range Shh enhancer regulates expression in the developing limb and fin and is associated with preaxial polydactyly. Hum. Mol. Genet. 12, 1725–1735 (2003).
Cho, T. J., Baek, G. H., Lee, H. R., Moon, H. J., Yoo, W. J. & Choi, I. H. Tibial hemimelia-polydactyly-five-fingered hand syndrome associated with a 404 G>A mutation in a distant sonic hedgehog cis-regulator (ZRS): a case report. J. Pediatr. Orthop. B 22, 219–221 (2013).
Chan, K. M. & Lamb, D. W. Triphalangeal thumb and five-fingered hand. Hand 15, 329–334 (1983).
Vargas, F. R., Pontes, R. L., Llerena Junior, J. C. & de Almeida, J. C. Absent tibiae—polydactyly—triphalangeal thumbs with fibular dimelia: variable expression of the Werner (McKusick 188770) syndrome? Am. J. Med. Genet. 55, 261–264 (1995).
Kantaputra, P. N. & Chalidapong, P. Are triphalangeal thumb-polysyndactyly syndrome (TPTPS) and tibial hemimelia-polysyndactyly-triphalangeal thumb syndrome (THPTTS) identical? A father with TPTPS and his daughter with THPTTS in a Thai family. Am. J. Med. Genet. 93, 126–131 (2000).
Agarwal, R. P., Jain, D., Ramesh Babu, C. S. & Garg, R. K. A hereditable combination of congenital anomalies. J. Bone Joint Surg. Br. 78, 492–494 (1996).
Afshar, A. A rare syndrome of five finger hands and polydactyly of the feet: a case report. J. Hand Microsurg. 3, 86–88 (2011).
Lettice, L. A., Williamson, I., Wiltshire, J. H., Peluso, S., Devenney, P. S., Hill, A. E. et al. Opposing functions of the ETS factor family define Shh spatial expression in limb buds and underlie polydactyly. Dev. Cell 22, 459–467 (2012).
Zhao, J., Ding, J., Li, Y., Ren, K., Sha, J., Zhu, M. et al. HnRNP U mediates the long-range regulation of Shh expression during limb development. Hum. Mol. Genet. 18, 3090–3097 (2009).
Lettice, L. A., Hill, A. E., Devenney, P. S. & Hill, R. E. Point mutations in a distant sonic hedgehog cis-regulator generate a variable regulatory output responsible for preaxial polydactyly. Hum. Mol. Genet. 17, 978–985 (2008).
Sagai, T., Masuya, H., Tamura, M., Shimizu, K., Yada, Y., Wakana, S. et al. Phylogenetic conservation of a limb-specific, cis-acting regulator of Sonic hedgehog (Shh). Mamm. Genome 15, 23–34 (2004).
Masuya, H., Sezutsu, H., Sakuraba, Y., Sagai, T., Hosoya, M., Kaneda, H. et al. A series of ENU-induced single-base substitutions in a long-range cis-element altering Sonic hedgehog expression in the developing mouse limb bud. Genomics 89, 207–214 (2007).
Yurkiewicz, I. R., Korf, B. R. & Lehmann, L. S. Prenatal whole-genome sequencing—is the quest to know a fetus’s future ethical? New Engl. J. Med. 370, 195–197 (2014).
Acknowledgements
This study was supported by a grant from the Ratchadapiseksomphot Endowment Fund of Chulalongkorn University (CU) (RES560530177-HR), the Royal Golden Jubilee and CU (PHD/0003/2556) and the Thailand Research Fund (RTA5680003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Norbnop, P., Srichomthong, C., Suphapeetiporn, K. et al. ZRS 406A>G mutation in patients with tibial hypoplasia, polydactyly and triphalangeal first fingers. J Hum Genet 59, 467–470 (2014). https://doi.org/10.1038/jhg.2014.50
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhg.2014.50
This article is cited by
-
A novel smoothed (SMO) point mutation in congenital tibial hemimelia: a case report
BMC Pediatrics (2023)