Abstract
Norvancomycin (NVCM) is widely used in China to treat bacterial infections of Gram-positive cocci and bacilli, especially infections of methicillin-resistant Staphylococcus aureus and methicillin-resistant Staphylococcus epidermidis. This study is a chemical investigation of the drug substance of NVCM, and led to the identification, by 1D, 2D NMR spectra and HRESIMS, of three as yet undescribed impurities, one D-O-E ring expanded NVCM analog (1) and two derivatives of NVCM lacking sugars (2, 3).
Similar content being viewed by others
Introduction
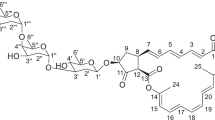
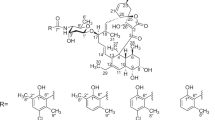
Norvancomycin (NVCM), a glycopeptide antibiotic, was commercially developed in China and has been widely used to treat endocarditis, osteomyelitis and other severe infections for over three decades.1 It is active against Gram-positive bacteria, especially methicillin-resistant Staphylococcus aureus and methicillin-resistant S. epidermidis, as well as penicillin-resistant Corynebacterium jeikeium, Streptococcus pneumoniae, and Clostridium difficile. NVCM was produced by a microorganism (Amycolatopsis orientalis) isolated from soil samples from Guizhou Province, China, in 1959.2 The structure of NVCM closely resembles that of the well-known glycopeptide antibiotic vancomycin (VCM), and the in vitro and in vivo activities of NVCM were similar to that of VCM.3 NVCM consists of a tricyclic central core of heptapeptide and a disaccharide part as does VCM, only lacking an N-methyl group at the N-terminus of the polypeptide (Figure 1). In the literature, the seven amino acids of the heptapeptide were designated as AA-1 to AA-7, the five aromatic rings were lettered A through E, and the three larger rings (corresponding to the tricyclic central core) took the letters of their component aryl rings, A-B, C-O-D and D-O-E, respectively.
Early VCM products were reported to be associated with many side effects such as infusion reactions, nephrotoxicity and ototoxicity, most likely due to impurities in VCM drug substance. The impurities in VCM drug substance have been intensively investigated by high-performance liquid chromatography (HPLC),4, 5, 6, 7 capillary electrophoresis8 and liquid chromatography–MS/MS.4, 9 More than eight impurities were isolated from VCM drug substance, including monodechlorovancomycin A and B,10 didechlorovancomycin,10 crystalline degradation products (CDP-IM and CDP-Im),11 aglucovancomycin,12 desvancosaminylvancomycin12 and NVCM. Of them, the monodechlorovancomycin A and B, and the didechlorovancomycin were reported to possess 2–10 folds lower activities than VCM.3 In addition, the crystalline degradation products, CDP-IM and CDP-Im, have been shown to cause adverse effects and be associated with the therapeutic failure of VCM.13 Desvancosaminylvancomycin showed a dramatic decrease (five-fold) in in vivo antibacterial activity compared with VCM.3 Desvancosaminylvancomycin, aglucovancomycin and NVCM are three main controlled impurities of VCM hydrochloride drug substance in the European Pharmacopoeia14 and United States Pharmacopoeia,15 and are limited to ⩽7.0% total and ⩽4.0% for each.
In the Chinese Pharmacopoeia,16 the impurities for NVCM hydrochloride drug substance are limited to ⩽12.0% total and ⩽4.0% for each. However, the chemical structures of impurities in NVCM drug substance have still not been determined. In this paper, we reported the purification and chemical structure determination of three impurities from NVCM drug substance. These are NVCM derivatives, one with D-O-E ring expansion (1) and two lacking sugars (2, 3). Their structures were elucidated from extensive spectroscopic one-dimensional and two-dimensional (1D and 2D) NMR analysis, as well as high resolution electrospray ionization mass spectroscopy (HRESIMS).
Results
Isolation and purification of impurities in NVCM drug substance
The HPLC method described in the Chinese Pharmacopoeia was initially used to analyze NVCM drug substance for impurities (Figures 2a and b). A predominant peak (95.50% by area) corresponding to NVCM appeared at 21.44 min. More than three small peaks were eluted before or after the predominant peak. These small peaks represent impurities in NVCM drug substance. However, they were not suitable for liquid chromatography–MS analysis due to phosphate residue from the mobile phase.
Therefore, a new HPLC method was developed using a salt-free MeOH-water mixture with 0.1% formic acid as the mobile phase (see Material and methods section). The new method generated four sharp minor peaks, together with the predominant NVCM peak (Figures 2c and d). The four minor peaks accounted for 3.46% (1), 1.91% (2), 0.96% (3) and 2.02% (4), respectively, while the dominant NVCM peak occupied 87.97% by peak area.
The salt-free mobile phase was also used for HPLC preparation of impurities 1, 2 and 3 (HPLC spectrum see Supplementary Figure S1). From 5.0 g NVCM drug substance, we obtained 5.0 mg impurity 1, 5.0 mg impurity 2, 1.5 mg impurity 3, as well as 4.5 g purified NVCM (>95%). Unfortunately, we did not get an enough amount of impurity 4 (corresponding to peak 4, very close to the NVCM peak) for structure elucidation, as repeated preparative HPLCs were necessary for its complete separation from the main component NVCM, and this dramatically reduced its yield.
Structure elucidation of the impurities
The physico-chemical properties of impurities 1, 2 and 3 are presented in the Supplementary Material. Impurities 1, 2 and 3 displayed very similar UV spectra to NVCM (Figure 2d, Supplementary Figures S2–S5), suggested that they may be NVCM analogs. Impurity 1 was obtained as a colorless gum with [α]20D −44.5 (c 0.22, MeOH). The electrospray ionization mass spectroscopy (ESIMS) and HRESIMS ([M+2H]2+ ion at 724.7503) indicated its molecular formula to be C66H75O24N9Cl2 (Supplementary Figures S6). Impurity 1 should be a methylated derivative of NVCM, as its molecular formula is one CH2 more than that of NVCM (C65H73O24N9Cl2). A similar mass difference was also observed between the product ions of 1 (at m/z 1305 and 1143, Figure 3c) and NVCM (at m/z 1,291 and 1,129, Figure 3a) in their ESIMS/MS spectra. A detailed analysis of the fragmentation pathway of NVCM (Figure 3b) and 1 (Figure 3d) suggested that impurity 1 had the same vancosaminyl-O-glucosyl disaccharide group as NVCM. Therefore, the methyl group must be located within the heptapeptide core of 1.
The ESIMS/MS spectra of norvancomycin (NVCM; a, the parent ion [M+H]+ m/z 1434) and impurity 1 (c, the parent ion [M+2H]2+ m/z 724.5), and the fragmentation pathways (b for NVCM, d for impurity 1, respectively). The lost fragments m/z 305 (F1) and 143 (F2) are corresponding to vancosaminyl-O-glucosyl disaccharide and vancosamine, respectively.
The amino acid residues of the heptapeptide core in NVCM, including three phenylglycines, one leucine, two 3,4-disubstituted phenylalanines, and one asparagine or aspartic acid moieties, appear to be present in 1 based on 1D and 2D NMR spectra (Supplementary Figures S8–S13). A further comparison of the NMR data of 1 with those of NVCM in the same solvent (Table 1, Figure 4) revealed that their chemical shift differences focused on one phenylglycine (AA-2) and the asparagine (AA-3) in ring D-O-E. The signals of H-(AA-3)-2, and H-(AA-3)-3a were shielded by ΔδH −0.37 and –0.12 p.p.m., respectively, and the signal of H-(AA-3)-3b was deshielded by ΔδH +0.4 p.p.m. These chemical shift values for asparagine were, in fact, very similar to those in CDP-I (including CDP-IM and CDP-Im, different at the orientation of C–Cl bond at ring E),11 rearrangement products with D-O-E expansion generated in the crystallization process of VCM. Especially, in the nuclear Overhauser effect spectroscopy (NOESY) spectrum (Supplementary Figure S34), H2-(AA-3)-3 showed correlations to H-(AA-2)-5, H-(AA-3)-2, H-(AA-2)-6 and H-(AA-2)-5 to H-(AA-4)-2, which suggested that these protons were oriented on the same side of the ring system. In addition, the correlations of H-(AA-2)-2 with H-(AA-2)-7, H-(AA-2)-8 and H-(AA-4)-6, and H-(AA-4)-6 with H-(AA-4)-7, indicated that these protons were oriented on the other side of the ring system, and the C–Cl bond at ring E kept the same orientation with that of NVCM, VCM and CDP-IM.11 The 1D and 2D NMR data (Table 1, Figure 4, Supplementary Figures S8–S13) of impurity 1 suggested that 1 had the same configuration concerning amino acids AA-5, AA-6 and AA-7, and conformation about rings A–B and C-O-D.
The methyl group mentioned above was assigned to an N-CH3 group as an additional single peak (δH 3.17 p.p.m. (3H, s) and δC 48.5 p.p.m.) appeared in the NMR of impurity 1. This N-CH3 group was further assigned to the N-terminal amino acid residue leucine based on an liquid chromatography–MS analysis of the alkaline hydrolysis products of impurity 1, VCM and NVCM (Supplementary Material, Supplementary Figures S38–S40). The result showed that an m/z 146 [M+H]+ ion corresponding to N-methyl leucine appeared in the hydrolytic products of impurity 1, resembled that of VCM. Therefore, the structure of impurity 1 was determined as the D-O-E expanded analog of N-methyl-NVCM.
The spectroscopic data (Supplementary Figures S3) of impurity 2 indicated that it was also an analog of NVCM. A comparison of the NMR data of 2 (Table 2) with those of NVCM indicated that the vancosaminyl-O-glucosyl group in NVCM was replaced by a glucosyl group in 2. In particular, 2 lost the specific methyl signals of the vancosaminyl group in NVCM at δH 1.29 (s), and 1.06 (d, J=6.6 Hz), and its anomeric proton signal at δ 5.23 (m). In addition, the carbon signal of C-2 of the glucosyl group was shielded by ΔδC–4.4 p.p.m. These data, together with a 143 atomic units (equivalent to a group of C7H13NO3 for vancosaminyl) less than NVCM in molecular mass by HRESIMS, supported the formulation of 2 as desvancosaminylnorvancomyicn.
The UV (Supplementary Figure S4) and NMR (Supplementary Figures S23,S24,S26) spectra of 3 were similar to those of 2. A careful comparison of their NMR data indicated that 3 was a deglucosyl analog of 2. The ESIMS and ESIMS/MS spectra of 3 are displayed in the Supplementary Material (Supplementary Figures S25). In addition, the HRESIMS (Supplementary Figure S26) of 3 showed a pseudomolecular ion peak of [M+H]+ at m/z 1129.2783 (calculated for molecular formula C52H50O17N8Cl2), which was 162 atomic units (equivalent to a group of C6H10O5 for glucosyl) less than that of 2. Thus, the chemical structure of impurity 3 was determined as the aglycone of NVCM (Figure 1). The ESIMS and NMR spectra of NVCM are displayed in the Supplementary Material (Supplementary Figures S28–S33) for comparison.
Antibacterial activity
The in vitro antibacterial activities of impurities 1–3 were evaluated, together with purified NVCM (purity >95%), NVCM drug substance and standard VCM (Table 3). Impurity 1 lost almost all activities against the test bacterial strains. Impurity 2 showed 2–5 folds lower activity than NVCM. Impurity 3 displayed similar activity to NVCM. The purified NVCM, NVCM drug substance and VCM exhibited almost the same activity. As expected, impurities 1–3, together with NVCM and VCM, showed no activity against Escherichia coli and most of the VCM-resistant enterococci.
Discussion
In the current Chinese Pharmacopeia,16 NVCM hydrochloride drug substance is specified to contain NVCM⩾88%, and each impurity ⩽4%. But the structures of impurities in NVCM hydrochloride drug substance are not specified in the Chinese Pharmacopeia as no such data were available. Therefore, it is necessary to characterize the impurities in NVCM drug substance, which should be not only essential in promoting the establishment of a revised quality control standard of NVCM drug substance for the Chinese Pharmacopeia, but also helpful in the accurate quality assessment of NVCM drug substance.
In this paper, a salt-free HPLC method was reported for analysis and purification of impurities in NVCM drug substance. Three NVCM-related impurities were obtained from NVCM drug substance by this method. Their chemical structures were determined to be a D-O-E expanded analog of N-methyl NVCM and two others lacking sugars. It is worth noting that the two corresponding derivatives of de-saccharide VCM (desvancosaminylvancomycin and aglucovancomycin) were also present as the two main controlled impurities in VCM drug substance.
Although the biosynthetic pathway of NVCM has not been reported yet, the draft genome of NVCM-producing strain17 showed that the gene cluster responsible for NVCM biosynthesis was highly similar to that responsible for the biosynthesis of VCM.18 As glycosylation is believed to be the last modification reaction after the heptapeptide core has been assembled in the VCM biosynthetic pathway,18 the two de-saccharide derivatives in NVCM and VCM drug substance might come from incomplete glycosylation(s) in the biosynthesis process.
The structure of impurity 1 is very similar to CDP-IM, but has a CONH2, not a COOH, at AA-3. Impurity 1 might be a rearrangement product of N-methyl NVCM, which undergoes a seco D-O-E ring succinimide intermediate, and then the resulting free NH2 group of AA-4 attacks selectively C-4 of succinimide ring (producing an amide bond with AA-3) to form impurity 1 (Supplementary Scheme S1). This rearrangement mechanism is similar to CDP-I from VCM, except that an in-chain succinimide intermediate is hydrolyzed at C-1 of the succinimide ring, and generates a free COOH group in CDP-I.11 The N-CH3 group of impurity 1 might be generated by the expression of the N-methylase gene which was present in the biosynthetic gene cluster of NVCM.17 Impurity 1 lost the antibacterial activity near completely, consistent with the previous studies that three-dimensional structural integrity of the tricyclic heptapeptide core in VCM played a pivotal role in antibacterial activity, as the D-O-E expanded analog of NVCM could not match to the active site of its target.11, 19
Herein, three impurities of related structures from NVCM drug substance were reported for the first time. The impurity profile provides a preliminary basis for the establishment of an advanced quality control standard for NVCM. It may also offer valuable comprehensive indicator for the optimization of the fermentation and purification process in the manufacture of NVCM, and helps to improve the final product quality of NVCM drug substance.
Conclusions
In this work, an optimized HPLC method was developed for NVCM impurity analysis and preparation. Three NVCM-related impurities were identified by extensive spectroscopic 1D and 2D NMR and HRESIMS analysis. Their chemical structures were determined as one D-O-E ring expanded analog of N-methyl NVCM and two NVCM analogs lacking sugar moieties. The expansion of the D-O-E ring resulted in a significant decrease in antibacterial potency.
Materials and methods
Chemicals and reagents
Drug substance of NVCM hydrochloride was from New Drug Research and Development Center, North China Pharmaceutical Group Corporation, Shijiazhuang, China. Standard VCM was from National Institutes for Food and Drug Control, Beijing, China. Acetonitrile and MeOH of chromatographic pure grade were from Adamas Reagent Co., Ltd. Shanghai, China. DMSO-d6 was from Cambridge Isotope Laboratories, Inc., Cambridge, MA, USA. All other reagents (HCOOH, H3PO4, hydrochloric acid, trifluoroacetic acid, NH3·H2O (NH3, 25–28% in mass fraction), triethylamine and tetrahydrofuran) were of analytical reagent grade.
Instruments
The following instruments were used in this study: a P-2000 polarimeter (JASCO, Tokyo, Japan), a JASCOP-650 spectrometer (JASCO), SYS 600 MHz NMR spectrometers (Palo Alto, CA, USA), a Finnigan LTQ XT ion trap mass spectrometer (Finnigan, San Jose, CA, USA), a Finnigan LTQ Orbitrap XT mass spectrometer (Finnigan), an HPLC system consisting of a Shimadzu LC-20AP pump (Shimadzu Corporation, Kyoto, Japan), and a SPD-20AV detector (Shimadzu).
Analytical and preparative HPLC spectrometry
Method in Chinese Pharmacopoeia
Welchrom C18 column (250 × 4.6 mm i.d., 5 μm), triethylamonium phosphate water solution (adjusted to pH 3.2 by phosphoric acid)—acetonitrile—tetrahydrofuran (96:3:1) as mobile phase, where a small quantity of acetonitrile was used to adjust the main peak at 18–22 min. 1.0 ml min−1, detection wavelength 280 nm, room temperature (25 °C).16 A sample volume of 20 μl of NVCM drug substance in water (1.0 mg ml−1) was injected.
Optimized analytical HPLC method
Agilent C18 column (150 × 4.6 mm i.d., 5 μm), MeOH-H2O with 0.1% (v/v) HCOOH as mobile phase, a linear increase of MeOH from 10% to 70% in 40 min, 1.0 ml min−1, detection wavelength 280 nm, room temperature (25 °C). A sample volume of 20 μl NVCM drug substance in water (3.0 mg ml−1) was injected.
Preparative HPLC
A YMC-Pack Ph column (150 × 20 mm i.d., 20 μm, YMC Co., Ltd, Kyoto, Japan) was used for the preparation of impurities. The mobile phase was the same as the optimized analytical HPLC method, but the flow rate was 5.0 ml min−1 and the elution time was 120 min. A total 300 μl of NVCM drug substance in water (100 mg ml−1) was injected.
Chemical structure elucidation
NMR spectra were obtained at 600 MHz for 1H, and 150 MHz for 13C, respectively, on SYS 600 spectrometers (Palo Alto, CA, USA) in DMSO-d6 with a low temperature probe. The 1H and 13C chemical shift values are reported on the δ scale in p.p.m. relative to TMS (δ=0.00 p.p.m.) and DMSO-d6 (δ=39.5 p.p.m.) as the internal standards, respectively.
Antibacterial assays
The MICs were determined by using the agar dilution method described by the Clinical and Laboratory Standards Institute.20 VCM was used as the positive control, and the test bacteria were ATCC standard strains or clinical isolates. Muller-Hinton broth was used as the test medium and the inoculum was 10 000 CFU/spot. The final concentrations were 0.5 to 128 μg ml−1 for each sample. Culture plates were incubated at 35 °C for 18 h, and the lowest concentration that prevented visible growth of bacteria was recorded as the MIC.
References
Wu, X. J. et al. Establishment of norvancomycin fluorescence polarization immunoassay for therapeutic drug monitoring. J. Antibiot. (Tokyo) 65, 35–39 (2012).
Li, Q., Song, A., Liu, J. R. & Wang, X. Y. Actinomycetes Van 23-Vancomycin Producing Strain, (Shanghai scientific & Technical Publishers, Shanghai, China, (1962).
Nagarajan, R. Structure-activity relationships of vancomycin-type glycopeptide antibiotics. J. Antibiot. (Tokyo) 46, 1181–1195 (1993).
Diana, J., Visky, D., Hoogmartens, J., Van Schepdael, A. & Adams, E. Investigation of vancomycin and related substances by liquid chromatography/ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 20, 685–693 (2006).
Diana, J., Visky, D., Roets, E. & Hoogmartens, J. Development and validation of an improved method for the analysis of vancomycin by liquid chromatography selectivity of reversed-phase columns towards vancomycin components. J. Chromatogr. A 996, 115–131 (2003).
Inman, E. L. Determination of vancomycin related substances by gradient high-performance liquid chromatography. J. Chromatogr. 410, 363–372 (1987).
Jesus Valle, M. J., Lopez, F. G. & Navarro, A. S. Development and validation of an HPLC method for vancomycin and its application to a pharmacokinetic study. J. Pharm. Biomed. Anal. 48, 835–839 (2008).
Kang, J. W., Van Schepdael, A., Roets, E. & Hoogmartens, J. Analysis of vancomycin and related impurities by micellar electrokinetic capillary chromatography. Method development and validation. Electrophoresis 22, 2588–2592 (2001).
Hadwiger, M. E., Sommers, C. D., Mans, D. J., Patel, V. & Boyne, M. T. 2nd Quality assessment of U.S. marketplace vancomycin for injection products using high-resolution liquid chromatography-mass spectrometry and potency assays. Antimicrob. Agents Chemother. 56, 2824–2830 (2012).
Harris, C. M., Kannan, R., Kopecka, H. & Harris, T. M. The role of the chlorine substituents in the antibiotic vancomycin: preparation and characterization of mono- and didechlorovancomycin. J. Am. Chem. Soc. 107, 6652–6658 (1985).
Harris, C. M., Kopecka, H. & Harris, T. M. Vancomycin: structure and transformation to CDP-I. J. Am. Chem. Soc. 105, 6915–6922 (1983).
Nagarajan, R. & Schabel, A. A. Selective cleavage of vancosamine, glucose, and N-methyl-leucine from vancomycin and related antibiotics. J. Chem. Soc. Chem. Commun. 19, 1306–1307 (1988).
Somerville, A. L., Wright, D. H. & Rotschafer, J. C. Implications of vancomycin degradation products on therapeutic drug monitoring in patients with end-stage renal disease. Pharmacotherapy 19, 702–707 (1999).
The European Pharmacopoeia Commission The European Pharmacopoeia, 8th edn, 3525–3527 (Council of Europe, Strasbourg, France, (2014).
The United States Pharmacopoeia Commission Inc. The United States Pharmacopeia,, USP28-NF23, Vol. 27, 2784 (The United States Pharmacopeia Convention, Inc, Rockville, MD, (2005).
Chinese Pharmacopoeia Commission. Chinese Pharmacopoeia of the People's Republic of China (in Chinese) 2015 edn, Part II, 881 China Medical Science Press, Beijing, China, (2015).
Lei, X. et al. Draft genome sequence of norvancomycin-producing strain amycolatopsis orientalis CPCC200066. Genome Announc. 3, e00296-15 (2015).
Xu, L. et al. Complete genome sequence and comparative genomic analyses of the vancomycin-producing Amycolatopsis orientalis. BMC Genomics 15, 363 (2014).
Loll, P. J., Miller, R., Weeks, C. M. & Axelsen, P. H. A ligand-mediated dimerization mode for vancomycin. Chem. Biol. 5, 293–298 (1998).
Wikler, M. A. Clinical and Laboratory Standards Institute Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard-8th ed. Report No. 12M07-A7 (2009).
Acknowledgements
This work was supported by the National Mega-Project for Innovative Drugs (2014ZX09201001-004-001 and 2012ZX09301002-001-016) and the National Natural Science Foundation of China (81321004, 81302677, 81402836 and 31170042).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on The Journal of Antibiotics website
Supplementary information
Rights and permissions
About this article
Cite this article
Jiang, Z., Lei, X., Chen, M. et al. Three structurally-related impurities in norvancomycin drug substance. J Antibiot 70, 158–165 (2017). https://doi.org/10.1038/ja.2016.115
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2016.115
This article is cited by
-
Dolyemycins A and B, two novel cyclopeptides isolated from Streptomyces griseus subsp. griseus HYS31
The Journal of Antibiotics (2018)