Abstract
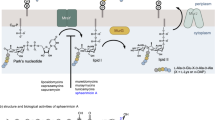
Vicenistatin (1) is a 20-membered polyketide macrocyclic antibiotic with potent antimicrobial and cytotoxic activities. In this study, to further explore the potential of 1 as candidates of antibacterial drug development, 4’-N-demethyl vicenistatin (2), a secondary metabolite obtained from the ∆vicG mutant strain of Monodonata labio-associated Streptomyces parvus SCSIO Mla-L010, was utilized as a starting material for modifications of 4’-amino group of vicenistatin. Six new vicenistatin derivatives (3–8) were semi-synthesized through a concise route of amino modification with various aliphatic and aromatic aldehydes. Our study reveals that the bioactivity of vicenistatin is closely related to amino modification in sugar moiety, which results from the length of alkyl side chain as well as the presence of electron withdrawing/denoting group on the benzene ring. Importantly, compounds 4 with a butyl group and 8 with a 3,5-dihydroxyl-benzyl group at 4’-amino group, respectively, exhibited good antimicrobial activities, with MIC values spanning 0.5–4 μg ml−1 to Gram-positive pathogens, including methicillin-resistant Staphylococcus aureus, methicillin-resistant Staphylococcus epidermidis, Micrococcus luteus and Bacillus subtilis, with low cytotoxicity. This research promotes the further exploration of structure-activity relationships of vicenistatin and provides new vicenistatin derivatives for development of future anti-infectious agents with reduced cytotoxicity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Shindo K, Kamishohara M, Odagawa A, Matsuoka M, Kawai H. Vicenistatin, a novel 20-membered macrocyclic lactam antitumor antibiotic. J Antibiot. 1993;46:1076–81.
Matsushima Y, Itoh H, Eguchi T, Kakinuma K. Enantioselective and convergent synthesis of the 20-membered lactam aglycon of vicenistatin antitumor antibiotic. J Antibiot. 1998;51:688–91.
Matsushima Y, Itoh H, Nakayama T, Horiuchi S, Eguchi T, Kakinuma K. Enantioselective total synthesis of vicenistatin, a novel 20-membered macrocyclic lactam antitumor antibiotic. J Chem Soc, Perkin Trans 1. 2002;7:949–58.
Fukuda H, Nakamura S, Eguchi T, Iwabuchi Y, Kanoh N. Concise total synthesis of vicenistatin. Synlett. 2010;17:2589–92.
Fukuda H, Nishiyama Y, Nakamura S, Ohno Y, Eguchi T, Iwabuchi Y, Usui T, Kanoh N. Synthesis and structure–activity relationship of vicenistatin, a cytotoxic 20‐membered macrolactam glycoside. Chem Asian J. 2012;7:2872–81.
Nishiyama Y, Ohmichi T, Kazami S, Iwasaki H, Mano K, Nagumo Y, Kudo F, Ichikawa S, Iwabuchi Y, Kanoh N, Eguchi T, Osada H, Usui T. Vicenistatin induces early endosome-derived vacuole formation in mammalian cells. Biosci Biotech Bioch. 2016;80:902–10.
Ogasawara Y, Katayama K, Minami A, Otsuka M, Eguchi T, Kakinuma K. Cloning, sequencing, and functional analysis of the biosynthetic gene cluster of macrolactam antibiotic vicenistatin in Streptomyces halstedii. Chem Biol. 2004;11:79–86.
Ogasawara Y, Kakinuma K, Eguchi T. Involvement of glutamate mutase in the biosynthesis of the unique starter unit of the macrolactam polyketide antibiotic vicenistatin. J Antibiot. 2005;58:468–472.
Minami A, Uchida R, Eguchi T, Kakinuma K. Enzymatic approach to unnatural glycosides with diverse aglycon scaffolds using glycosyltransferase VinC. J Am Chem Soc. 2005;127:6148–9.
Kudo F, Kitayama T, Kakinuma K, Eguchib T. Macrolactam formation catalyzed by the thioesterase domain of vicenistatin polyketide synthase. Tetrahedron Lett. 2006;47:1529–32.
Minami A, Eguchi T. Substrate flexibility of vicenisaminyltransferase VinC involved in the biosynthesis of vicenistatin. J Am Chem Soc. 2007;129:5102–7.
Shinohara Y, Kudo F, Eguchi T. A natural protecting group strategy to carry an amino acid starter unit in the biosynthesis of macrolactam polyketide antibiotic. J Am Chem Soc. 2011;133:18134–7.
Shinohara Y, Miyanaga A, Kudo F, Eguchi T. The crystal structure of the amidohydrolase VinJ shows a unique hydrophobic tunnel for its interaction with polyketide substrates. FEBS Lett. 2014;588:995–1000.
Miyanaga A, Ciesak J, Shinohara Y, Kudo F, Eguchi T. The crystal structure of the adenylation enzyme VinN reveals a unique β-amino acid recognition mechanism. J Biol Chem. 2014;289:31448–57.
Miyanaga A, Iwasawa S, Shinohara Y, Kudo F, Eguchi T. Structure-based analysis of the molecular interactions between acyltransferase and acyl carrier protein in vicenistatin biosynthesis. Proc Natl Acad Sci USA. 2016;113:1802–7.
Chisuga T, Miyanaga A, Eguchi T. Protein‐protein recognition involved in the intermodular transacylation reaction in modular polyketide synthase in the biosynthesis of vicenistatin. ChemBioChem. 2022;23:e202200200.
Miyanaga A, Kawada K, Chisuga T, Kudo F, Eguchi T. Structural basis of transient interactions of acyltransferase vink with the loading acyl carrier protein of the vicenistatin modular polyketide synthase. Biochemistry. 2023;62:17–21.
Murray CJ, Ikuta KS, Sharara F, Swetschinski L, Robles Aguilar G, Gray A, Han C, Bisignano C, Rao P, Wool E, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399:629–55.
Liang Z, Li J, Ling C, Xu R, Yi X, Ju J, Li Q. Characterization of the aminosugar biosynthetic and regulatory genes of vicenistatin in monodonata labio-associated streptomyces parvus SCSIO Mla-L010. J Nat Prod. 2022;85:256–63.
Salta J, Reissig HU. Synthesis of divalent carbohydrate mimetics by reductive amination with enantiopure 1, 2-Oxazines as precursors. Synthesis. 2015;47:1893–98.
CLSI. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, approved standard, 8th ed.; Clinical and Laboratory Standards Institute: Villanova, PA; M07-A8.(2009).
Li W, Shi C, Wu X, Zhang Y, Liu H, Wang X, Huang C, Liang L, Liu Y. Light activation of iridium (III) complexes driving ROS production and DNA damage enhances anticancer activity in A549 cells. J Inorg Biochem. 2022;236:111977.
Acknowledgements
This work was supported in part by the National Natural Science Foundation of China (42006103), Guangzhou Basic and Applied Basic Research Foundation (202201010336; 202201010519; 2023A04J1162), the Key-Area Research and Development Program of Guangdong Province (2020B1111030005), Open Project of Guangdong Key Laboratory of Marine Materia Medica (LMM2021-6), the Natural Science Foundation of Guangdong (2021B1515020036), the Nansha District Science and Technology Plan Project (NSJL202102), and Guangdong Provincial University Young Innovative Talents Project (2021KQNCX036). We acknowledge Professor Minggui Wang from Huashan Hospital, Professors Xiaoxiao Liu and Bing Gu from Guangdong Provincial People’s Hospital for the gifts of pathogenic bacteria.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Yang, Z., Shi, C. et al. Semi-synthesis and structure-activity relationship study yield antibacterial vicenistatin derivatives with low cytotoxicity. J Antibiot 77, 221–227 (2024). https://doi.org/10.1038/s41429-023-00701-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-023-00701-3