Abstract
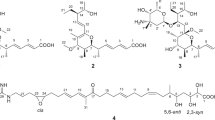
Four new yanuthone analogs (1–4) were isolated from the filamentous fungus Aspergillus niger. The structures of the new compounds were elucidated on the basis of UHPLC-DAD-HRMS data and one-dimensional and two-dimensional NMR spectroscopy. Labeling studies with 13C8-6-methylsalicylic acid identified three class I yanuthones originating from the polyketide 6-methylsalicylic acid (yanuthone K, L and M (1–3)) and a class II yanuthone, which was named yanuthone X2 (4). The four new compounds were tested toward the pathogenic yeast Candida albicans and all displayed antifungal activity. Yanuthone X2 represents the first example of a bioactive class II yanuthone, demonstrating the pharmaceutical potential of this class.
Similar content being viewed by others
Introduction
The black fungus Aspergillus niger is a significant contaminant of food and feeds, and a widely used cell factory for production of citric acid and bulk enzymes.1,2 In this context it is important to note that A. niger is known to produce >100 secondary metabolites,3 including not only mycotoxins such as ochratoxin A and fumonisins,3, 4, 5, 6, 7, 8 but also medically relevant compounds such as asperpyrone B and yanuthones targeting C. albicans.9,10 Recently, access to the full genome of A. niger11,12 has provided a platform that allows for linking genes to specific secondary metabolites. To this end, we have recently reported the characterization of the biosynthetic pathway toward antimicrobial yanuthone D in A. niger.13
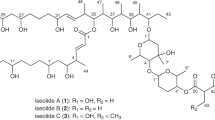
The core structure of yanuthones constitutes an epoxylated six-membered ring, which may be further decorated with different side chains: one sesquiterpene (at C13) and varying side chains (at C15 and C16), see Figure 1. The core structure may be derived from at least two different precursors, which lead to the formation of two classes of yanuthones. Class I yanuthones are derived from the polyketide 6-methylsalicylic acid (6-MSA), which due to a decarboxylation event during yanuthone formation, delivers a six-membered methylated ring (a C7 scaffold) to the yanuthones. Class II yanuthones contains a C6-core scaffold derived from an unknown precursor.13 The additional methyl group in class I yanuthones controls what type of decoration is possible at C16. Despite derivation of the two classes of yanuthones from different precursors, they share downstream enzymatic activities toward product maturation. So far, 15 class I yanuthones and a single class II yanuthone have been described in the literature of which only yanuthone D, a class I yanuthone, has displayed strong antimicrobial activity.9,13,14
These observations prompted us to investigate whether additional yanuthones exist in A. niger and whether such new compounds display antimicrobial activity. C. albicans accounts for the highest source of fungal infections worldwide,15 which is why it is of great interest to find new drug candidates. Here, we report the structures of four new yanuthones and their bioactivities toward C. albicans.
Results and Discussion
Identification and structural elucidation of four new yanuthones
Inspired by our previous discovery of six new yanuthones in A. niger,13 the A. niger strain KB1001 was cultivated on YES, and a large extract was scrutinized for compounds that could potentially be new yanuthones. The UHPLC-DAD-HRMS analysis tentatively identified four yanuthone-related structures. Their UV spectra as well as chemical compositions indicated coherence to the previously identified structures.9,13,14 The four compounds were purified and their structures elucidated from the HR-MS data, UV data and one-dimensional and two-dimensional (2D) NMR experiments (see Tables 1 and 2 and further details in the Supplementary Information).
All structures contain a six-membered ring containing an epoxide, which defines the core structure of yanuthones, but vary in their side chains, see Figure 1. Importantly, three of the compounds (yanuthones K–M (1–3)) contain a C7 core associated with class I yanuthones, whereas the remaining yanuthone X2 (4) contain a C6-core scaffold characteristic for class II yanuthone, hence representing the second compound of this class.
All four yanuthones showed several similar features in the 1H, as well as 2D spectra. All displayed eight overlapping proton resonances at δH=1.41–2.08 p.p.m. in the 1H spectrum, corresponding to the four methylene groups at C4, C5, C8 and C9, present in all structures. Other common resonances were seen for the diastereotopic pair H12/H12’ and the three methyl groups (H19, H20 and H21) around δH=1.60 p.p.m., of which H20 and H21 were overlapping. Moreover, all four yanuthones showed HMBC correlations to a quaternary carbon (C18) around δC=194 p.p.m., as well as two carbons around δC=60 p.p.m. (one quaternary, one methine) being the carbons in the epoxide ring.
The moiety attached to C15 varied, with yanuthone K–M having an acetoxy group attached (as also seen for yanuthone C9), whereas yanuthone X2 had a hydroxyl group (as seen in yanuthone A and E9). The structures also showed variance regarding the group attached to C16. Yanuthone K–M had a methyl group, whereas yanuthone X2 differed in having a methoxy group attached at the C16 position. This was obvious from the chemical shift of C16, which gave rise to a resonance at δC=172.6 p.p.m., considerably further downfield than in the other structures (yanuthone K–M). Furthermore, C17 was affected, shifting upfield to δC=98.8 p.p.m. As expected, the chemical shifts of the core structure of yanuthone K–M are very similar, whereas yanuthone X2 differs especially around position C16.
The attached terpene chain also varied in the four structures. Yanuthone K and X2 proved to contain a non-modified sesquiterpene. Yanuthone L and M also contain a sesquiterpene chain, but this is oxidized at C1 and C2, respectively.
NOESY experiments enabled determination of the stereogenic centers of 1–4 to be similar to the previously described yanuthones. For all four yanuthones the NOESY data showed a strong correlation between H14 and H15 (see Supplementary Information), suggesting these protons to be on the same side of the six-membered ring. This was further supported by the optical rotation measured, which was comparable to those reported for other yanuthones, altogether strongly indicating the absolute stereochemistry of C13, C14 and C15 is similar to other yanuthones.
Labeling studies confirm that yanuthones K–M are class I yanuthones, and yanuthone X2 is a class II yanuthone
In previous work it was demonstrated that yanuthone D, unlike the highly similar compound yanuthone X1, is biosynthesized from 6-MSA.13 To prove that yanuthones K, L and M truly are class I yanuthones and that yanuthone X2 is a class II yanuthone, the A. niger strain (KB1001) and a 6-MSA synthase deletion strain (yanAΔ)13 were analyzed for the presence of the four new yanuthones. This experiment revealed that neither yanuthone K, L nor M were present in yanAΔ (data not shown) suggesting that these three compounds are class I compounds, biosynthesized using 6-MSA as a precursor. In contrast, yanuthone X2 was still present in the yanAΔ strain, indicating that it is indeed a class II yanuthone. To further verify this, fully labeled 13C8-MSA was fed to yanAΔ and KB1001. Yanuthone L was not produced under the given conditions in this experiment, but the data confirmed that 6-MSA was indeed incorporated into yanuthone K and M, verifying 6-MSA as the precursor (Figure 2). In contrast, no 13C8-6-MSA was incorporated into yanuthone X2, which is in agreement with this compound being a class II yanuthone.
Yanuthones K–M and X2 display antifungal activity
Some of the yanuthones have earlier been reported to display antimicrobial activity,9 and in our previous study we reported that several of the yanuthones showed antifungal activity toward C. albicans, with yanuthone D being the most active with an IC50 value of 3.3 μM.13 We therefore tested the four new yanuthones identified in this study for antifungal activity toward C. albicans, see Table 3. All three class I yanuthones showed antifungal activity in this assay. The strongest activities were observed with yanuthones K and L, which resulted in IC50 values that were fivefold higher than that obtained with yanuthone D. The weakest activity was obtained with yanuthones M, which was 20-fold higher than the value obtained with yanuthone D. Importantly, yanuthone X2 also displayed an antimicrobial effect. With an IC50 value 15-fold higher than that of yanuthone D, it represents the first example of a bioactive class II yanuthone. This result demonstrates that there may be a pharmaceutical potential for this class of yanuthones.
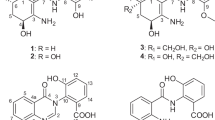
Comparison of all class I yanuthone structures to their corresponding antifungal activities revealed some structure–activity relationship features that may be relevant for potency toward C. albicans. First, yanuthone D (IC50=3.3 μM) and yanuthone E (IC50>100 μM) only differ by the functional group at C15. In this case, a shift from of a ketone to a hydroxyl at this position is sufficient to eliminate the antimicrobial effect of the most bioactive yanuthone (see Figure 3). Inspection of the other compounds revealed that the functional group at C15 does have a significant impact on the potency, as O-glycosylation increases antifungal activity (yanuthone G compared with yanuthone F), and O-acetylation at C15 also increases antifungal activity compared with hydroxylation (yanuthone K and L compared with 7-deacetoxyanuthone A and yanuthone F, respectively (7-deacetoxyyanuthone A was named by Li et al.14 using another numbering system for the yanuthones than the originally suggested by Bugni et al.9 According to the latter numbering system, which has also been used here, the appropriate name for 7-deacetoxyyanuthone A would have been 22-deacetoxyyanothone A).
Second, hydroxylation of C22 appears to increase antifungal activity. This is seen in yanuthone H (IC50=24.5 μM) compared with yanuthone F (IC50>100 μM), as well as in 22-deacetylyanuthone A (IC50=19.4 μM) compared with 7-deacetoxyyanuthone A (IC50>100 μM) (Figure 3). In both cases, the structures differ only by the C22 hydroxylation.
Last, two features seem to decrease antifungal activity: chain shortening of the sesquiterpene and hydroxylation of C2. Chain shortening of the sesquiterpene of yanuthone I (IC50>100 μM) decreased antifungal activity compared with yanuthone H (IC50=24.5 μM), which contains an intact C15 sesquiterpene (Figure 3). However, this could also be connected to the level of oxidation, and not the chain length alone. Hydroxylation of C2 also seems to decrease the activity, as yanuthone M (IC50=77.5 μM) is less active than yanuthone K (IC50=17.5 μM) (Figure 3). It is unclear how O-mevalonation at C22 affects the activity as both 7-deacetoxyyanuthone A (non-mevalonated) and yanuthone E (mevalonated) have IC50 values above 100 μM, and thus the activities were too low to be measured in this assay. The same goes for hydroxylation at C1, where 7-deacetoxyyanuthone A (non-hydroxylated) and yanuthone F (hydroxylated) have IC50 values above 100 μM. On the basis of the above correlation between structures and antifungal activities, we propose that antifungal activity of class I yanuthones may increase by combining (1) oxidation of the hydroxyl group at C15 with hydroxylation of C22 or (2) O-acetylation of C15 with hydroxylation of C22.
For class II yanuthones, the picture is different. In contrast to class I yanuthones, O-acetylation at C15 appears to reduce antifungal activity compared with hydroxylation (Figure 3). As class II yanuthones lack the C22 carbon and have a methoxy group attached to C16 instead, there may be interplay between the side groups, which may explain this difference. As yanuthone X2, but not yanuthone E, shows antimicrobial bioactivity, it would be of great interest to synthesize a class II yanuthone with a ketone at C15, to investigate whether this compound shows a stronger biological effect than yanuthone D.
In summary, a large-scale cultivation of A. niger has led to the discovery of four new yanuthones, which were all characterized by one-dimensional and 2D NMR spectroscopy. Labeling studies with 13C8-6-MSA and comparison of the chemical profile of the A. niger strain (KB1001) with a 6-MSA synthase gene deletion strain (yanAΔ) revealed three class I yanuthones (yanuthone K–M) originating from 6-MSA and one class II yanuthone (yanuthone X2) originating from a yet unknown precursor. These results were in agreement with their elucidated structures, with yanuthone K–M containing a C7-core scaffold and yanuthone X2 a C6-core scaffold.
Furthermore, we have tested the antifungal activity of the new yanuthones toward the pathogenic yeast C. albicans and found that all were active. This not only shed light on the structure–activity relationships that are relevant for the potency toward C. albicans, but also demonstrated the possible pharmaceutical potential of the class II yanuthones, as yanuthone X2 represents the first example of a bioactive class II yanuthone.
Methods
Strains, media and feeding experiments
The strains used for this study were the A. niger ATCC1015-derived KB100116 and the yanAΔ strain (6-MSA PKS deleted).13 Solid plates with YES media (CBS KNAW Fungal Biodiversity Centre, Utrecht, the Netherlands) were prepared as described by Frisvad and Samson,17 and feeding experiments with fully labeled 13C8-6-MSA were carried out as described by Holm et al.13
Chemical analysis of strains
Strains were cultivated on solid YES media at 25 °C for 5–7 days in the dark. Extraction of metabolites was performed as described by Smedsgaard.18 Analysis was performed using reversed-phase ultra-HPLC UV/Vis diode array detector high-resolution time-of-flight MS on a maXis G3 orthogonal acceleration quadrupole time-of-flight mass spectrometer (Bruker Daltonics, Bremen, Germany) as described by Holm et al.13
Preparative isolation of selected metabolites
A. niger (KB1001) was cultivated on 200 plates of YES medium at 30 °C for 5 days in the dark. Extraction, workup and fractionation were performed as described by Holm et al.13 Final purification was completed on a semi-preparative HPLC system, which was a Waters 600 Controller with a Waters 996 photodiode array detector (Waters, Milford, MA, USA). This was achieved using a Luna II C18 column (250 × 10 mm, 5 μm, Phenomenex, Torrance, CA, USA) and a flow rate of 4 ml min−1. Solvents used were acetonitrile (ACN) of HPLC grade and milliQ-water (Merck Millipore, Darmstadt, Germany), both with 50 p.p.m. trifluoroacetic acid. For the isolation of yanuthone L, M and X2, a gradient of 40–100% ACN in 20 min was used, yielding 2.0, 1.5 and 1.2 mg, respectively. For isolation of yanuthone K an isocratic run with 90% ACN for 15 min was used, yielding 6.7 mg.
NMR and structural elucidation
The 1D and 2D spectra were recorded on a Varian Unity Inova-500 MHz spectrometer (Varian, Palo Alto, CA, USA). Spectra were acquired using standard pulse sequences, and 1H spectra as well as DQF-COSY, NOESY, HSQC and HMBC spectra were acquired. The deuterated solvent was CD3CN-d3 and signals were referenced by solvent signals for CD3CN-d3 at δH=1.94 p.p.m. and δC=1.32/118.26 p.p.m. Chemical shifts are reported in p.p.m. (δ) and scalar couplings in hertz (Hz). The sizes of the J coupling constants reported in the tables are the experimentally measured values from the spectra. There are minor variations in the measurements that may be explained by the uncertainty of J. NMR data for all compounds including 1H and 2D spectra are found in Supplementary Information. Optical rotation was measured on a Perkin Elmer 321 Polarimeter (Perkin Elmer, Waltham, MA, USA).
Antifungal susceptibility testing
All compounds were screened for antifungal activity toward C. albicans (IBT 654) in accordance with the CLSI standards, as described by Holm et al.13
References
Schuster E, Dunn-Coleman NS, Frisvad JC & Van Dijck PWM . On the safety of Aspergillus niger—a review. Appl. Microbiol. Biotechnol. 59, 426–435 (2002).
Perrone G et al. Biodiversity of Aspergillus species in some important agricultural products. Stud. Mycol. 59, 53–66 (2007).
Nielsen KF, Mogensen JM, Johansen M, Larsen TO & Frisvad JC . Review of secondary metabolites and mycotoxins from the Aspergillus niger group. Anal. Bioanal. Chem. 395, 1225–1242 (2009).
Frisvad JC, Smedsgaard J, Samson RA, Larsen TO & Thrane U . Fumonisin B2 production by Aspergillus niger. J. Agric. Food Chem. 55, 9727–9732 (2007).
Mogensen JM, Frisvad JC, Thrane U & Nielsen KF . Production of Fumonisin B2 and B4 by Aspergillus niger on grapes and raisins. J. Agric. Food Chem. 58, 954–958 (2010).
Noonim P, Mahakarnchanakul W, Nielsen KF, Frisvad JC & Samson RA . Fumonisin B2 production by Aspergillus niger in Thai coffee beans. Food Addit. Contam. 26, 94–100 (2009).
Mogensen JM, Larsen TO & Nielsen KF . Widespread occurrence of the mycotoxin fumonisin B2 in wine. J. Agric. Food Chem. 58, 4853–4857 (2010).
Knudsen PB, Mogensen JM, Larsen TO & Nielsen KF . Occurrence of fumonisins B(2) and B(4) in retail raisins. J. Agric. Food Chem. 59, 772–776 (2011).
Bugni TS et al. Yanuthones: novel metabolites from a marine isolate of Aspergillus niger. J. Org. Chem. 65, 7195–7200 (2000).
Song YC et al. Endophytic naphthopyrone metabolites are co-inhibitors of xanthine oxidase, SW1116 cell and some microbial growths. FEMS Microbiol. Lett. 241, 67–72 (2004).
Baker SE Aspergillus niger genomics: past, present and into the future. Med. Mycol. 44, 17–21 (2006).
Pel HJ et al. Genome sequencing and analysis of the versatile cell factory Aspergillus niger CBS 513.88. Nat. Biotechnol. 25, 221–231 (2007).
Holm DK et al. Molecular and chemical characterization of the biosynthesis of the 6-MSA derived meroterpenoid yanuthone D in Aspergillus niger. Chem. Biol. 21, 519–529 (2014).
Li X, Choi HD, Kang JS, Lee C-O & Son BW . New polyoxygenated farnesylcyclohexenones, deacetoxyyanuthone A and its hydro derivative from the marine-derived fungus Penicillium sp. J. Nat. Prod. 66, 1499–1500 (2003).
Richards M & Edwards J . Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect. Control Hosp. Epidemiol. 21: 510–515 (2000).
Chiang Y-M et al. Characterization of a polyketide synthase in Aspergillus niger whose product is a precursor for both dihydroxynaphthalene (DHN) melanin and naphtho-γ-pyrone. Fungal Genet. Biol. 48, 430–437 (2011).
Samson RA, Houbraken J, Thrane U, Frisvad JC & Andersen B . Food and Indoor Fungi (CBS KNAW Fungal Biodiversity Centre, Utrecht, the Netherlands, 2010).
Smedsgaard J Micro-scale extraction procedure for standardized screening of fungal metabolite production in cultures. J. Chromatogr. A 760, 264–270 (1997).
Acknowledgements
The study was supported by grant 09-064967 from the Danish Council for Independent Research, Technology and Production Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on The Journal of Antibiotics website
Supplementary information
Rights and permissions
About this article
Cite this article
Petersen, L., Holm, D., Knudsen, P. et al. Characterization of four new antifungal yanuthones from Aspergillus niger. J Antibiot 68, 201–205 (2015). https://doi.org/10.1038/ja.2014.130
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2014.130
This article is cited by
-
Safety of the fungal workhorses of industrial biotechnology: update on the mycotoxin and secondary metabolite potential of Aspergillus niger, Aspergillus oryzae, and Trichoderma reesei
Applied Microbiology and Biotechnology (2018)
-
Global analysis of biosynthetic gene clusters reveals vast potential of secondary metabolite production in Penicillium species
Nature Microbiology (2017)