Abstract
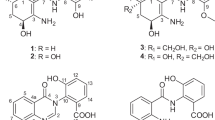
Two classes of new polyketides, allopteridic acids A–C (1–3) and allokutzmicin (4), were isolated from the culture extract of an actinomycete of the genus Allokutzneria. The structures of 1–4 were elucidated through the interpretation of NMR and MS analytical data. Compounds 1–3 possess the same carbon skeleton with pteridic acids but their monocyclic core structures are distinct from the spiro-bicyclic acetal structures of pteridic acids. Compound 4 is a linear polyketide of an unprecedented class, featured by a guanidino-terminus and an epoxide modification. Compounds 1–3 promoted the root elongation of germinated lettuce seeds by ca. 10–40% at 1~10 μM whereas 4 retarded the seed growth. Compound 4 exhibited weak antimicrobial activity against Candida albicans with MIC 25 μg mL−1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Becerril A, et al. Discovery of cryptic largimycins in Streptomyces reveals novel biosynthetic avenues enriching the structural diversity of the leinamycin family. ACS Chem Biol. 2020;15:1541–53.
He J, Van Treeck B, Nguyen HB, Melançon CE 3rd. Development of an unnatural amino acid incorporation system in the actinobacterial natural product producer Streptomyces venezuelae ATCC 15439. ACS Synth Biol. 2016;5:125–32.
Tiwari K, Gupta RK. Rare actinomycetes: a potential storehouse for novel antibiotics. Crit Rev Biotechnol. 2012;32:108–32.
Subramani R, Aalbersberg W. Culturable rare actinomycetes: diversity, isolation and marine natural product discovery. Appl Microbiol Biotechnol. 2013;97:9291–321.
Tiwari K, Gupta RK. Bioactive metabolites from rare actinomycetes. Stud Nat Prod Chem. 2014;41:419–512.
Hoshino S, Okada M, Awakawa T, Asamizu S, Onaka H, Abe I. Mycolic acid containing bacterium stimulates tandem cyclization of polyene macrolactam in a lake sediment derived rare actinomycete. Org Lett. 2017;19:4992–5.
Kurtböke DI. Biodiscovery from rare actinomycetes: an eco-taxonomical perspective. Appl Microbiol Biotechnol. 2012;93:1843–52.
Meier-Kolthoff JP, Sardà Carbasse J, Peinado-Olarte RL, Göker M. TYGS and LPSN: a database tandem for fast and reliable genome-based classification and nomenclature of prokaryotes. Nucleic Acids Res. 2022;50:D801–7.
Dictionary of Natural Products 30.2 Chemical Search. CRC Press, LLC. 2022. https://dnp.chemnetbase.com/faces/chemical/ChemicalSearch.xhtml.
Lauterbach L, Rinkel J, Dickschat JS. Two bacterial diterpene synthases from Allokutzneria albata produce bonnadiene, phomopsene, and allokutznerene. Angew Chem Int Ed Engl. 2018;57:8280–3.
Rinkel J, Lauterbach L, Rabe P, Dickschat JS. Two diterpene synthases for spiroalbatene and cembrene A from Allokutzneria albata. Angew Chem Int Ed Engl. 2018;57:3238–41.
Blin K, Shaw S, Kloosterman AM, Charlop-Powers Z, van Wezel GP, Medema MH, Weber T. AntiSMASH 6.0: Improving cluster detection and comparison capabilities. Nucleic Acids Res. 2021;49:W29–W35.
Biological Resource Center, NITE (NBRC) Home Page. https://www.nite.go.jp/nbrc/ (accessed 30 Dec 2022).
Igarashi Y, Iida T, Yoshida R, Furumai T. Pteridic acids A and B, novel plant growth promoters with auxin-like activity from Streptomyces hygroscopicus TP-A0451. J Antibiot. 2002;55:764–7.
Nong XH, Wei XY, Qi SH. Pteridic acids C-G spirocyclic polyketides from the marine-derived Streptomyces sp. SCSGAA 0027. J Antibiot. 2017;70:1047–52.
Garry RS, Robert M. Synthesis of methyl α-L-vancosaminide. Carbohydr Res. 1999;323:208–12.
Rubinstein E, Keynan Y. Vancomycin revisited-60 years later. Front Public Health. 2014;2:217.
Igarashi Y, et al. Clethramycin, a new inhibitor of pollen tube growth with antifungal activity from Streptomyces hygroscopicus TP-A0623. II. Physico-chemical properties and structure determination. J Antibiot. 2003;56:705–8.
Kobayashi J, Kubota T, Takahashi M, Ishibashi M, Tsuda M, Naoki H. Colopsinol A, a novel polyhydroxyl metabolite from marine dinoflagellate Amphidinium sp. J Org Chem. 1999;64:1478–82.
Corley DG, Moore RE, Paul VJ. Patellazole B: a novel cytotoxic thiazole-containing macrolide from the marine tunicate Lissoclinum patella. J Am Chem Soc. 1988;110:7920–2.
Du G, Tekin A, Hammond EG, Woo LK. Catalytic epoxidation of methyl linoleate. J Am Oil Chem Soc. 2004;81:477–80.
Matsumori N, Kaneno D, Murata M, Nakamura H, Tachibana K. Stereochemical determination of acyclic structures based on carbon-proton spin-coupling constants. A method of configuration analysis for natural products. J Org Chem. 1999;64:866–76.
Meissner A, Sørensen OW. Measurement of J(H,H) and long-range J(X,H) coupling constants in small molecules. Broadband XLOC and J-HMBC. Magn Reson Chem. 2001;39:49–52.
Dobashi K, Naganawa H, Takahashi Y, Takita T, Takeuchi T. Novel antifungal antibiotics octacosamicins A and B. II. The structure elucidation using various NMR spectroscopic methods. J Antibiot. 1988;41:1533–41.
Chandra A, Nair MG. Azalomycin F complex from Streptomyces hygroscopicus, MSU/MN-4-75B. J Antibiot. 1995;48:896–8.
Stubbendieck RM, Straight PD. Escape from lethal bacterial competition through coupled activation of antibiotic resistance and a mobilized subpopulation. PLoS Genet. 2015;11:e1005722.
Vandana UK, et al. The endophytic microbiome as a hotspot of synergistic interactions, with prospects of plant growth promotion. Biology. 2021;10:101.
Saito S, et al. A cyclopeptide and three oligomycin-class polyketides produced by an underexplored actinomycete of the genus Pseudosporangium. Beilstein J Org Chem. 2020;16:1100–10.
Saito S, Indo K, Oku N, Komaki H, Kawasaki M, Igarashi Y. Unsaturated fatty acids and a prenylated tryptophan derivative from a rare actinomycete of the genus Couchioplanes. Beilstein J Org Chem. 2021;17:2939–49.
Saito S, Oku N, Igarashi Y. Mycetoindole, an N-acyl dehydrotryptophan with plant growth inhibitory activity from an actinomycete of the genus Actinomycetospora. J Antibiot. 2022;75:44–7.
Lu S, Harunari E, Oku N, Igarashi Y. Trehangelin E, a bisacyl trehalose with plant growth promoting activity from a rare actinomycete Polymorphospora sp. RD064483. J Antibiot. 2022;75:296–300.
Saito S, et al. Phytohabitols A–C, δ-lactone-terminated polyketides from an actinomycete of the genus Phytohabitans. J Nat Prod. 2022;85:1697–703.
Liu C, et al. Catellatolactams A–C, plant growth-promoting ansamacrolactams from a rare actinomycete of the genus Catellatospora. J Nat Prod. 2022;85:1993–9.
Liu C, Yamamura H, Hayakawa M, Zhang Z, Oku N, Igarashi Y. Plant growth-promoting and antimicrobial chloropyrroles from a rare actinomycete of the genus Catellatospora. J Antibiot. 2022;75:655–61.
Zhang Z, et al. Kumemicinones A–G, cytotoxic angucyclinones from a deep sea-derived actinomycete of the genus Actinomadura. J Nat Prod. 2022;85:1098–108.
Harunari E, Mae S, Fukaya K, Tashiro E, Urabe D, Igarashi Y. Bisprenyl naphthoquinone and chlorinated calcimycin congener bearing thiazole ring from an actinomycete of the genus Phytohabitans. J Antibiot. 2022;75:542–51.
Karim MRU, Harunari E, Oku N, Akasaka K, Igarashi Y. Bulbimidazoles A–C, antimicrobial and cytotoxic alkanoyl imidazoles from a marine gammaproteobacterium Microbulbifer species. J Nat Prod. 2020;83:1295–9.
Sharma AR, Zhou T, Harunari E, Oku N, Trianto A, Igarashi Y. Labrenzbactin from a coral-associated bacterium Labrenzia sp. J Antibiot. 2019;72:634–9.
Takahashi N (ed.). Shokubutsu-kagaku-chosetsu-jikkenho (Experimental protocols for chemical regulation of plants). Tokyo: The Japanese Society for Chemical Regulation of Plants; 1989. p. 140–1 (in Japanese).
Acknowledgements
P388 cells were obtained from JCRB Cell Bank under an accession code JCRB0017 (Lot. 06252002). This work was supported by JSPS KAKENHI Grant Number 19K05848 to Y. I.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, C., Zhang, Z., Fukaya, K. et al. Isolation and structure determination of allopteridic acids A–C and allokutzmicin from an unexplored actinomycete of the genus Allokutzneria. J Antibiot 76, 305–315 (2023). https://doi.org/10.1038/s41429-023-00611-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-023-00611-4