Abstract
The discovery and characterization of natural congeners is one approach for understanding the relationship between chemical structure and biological function. We recently isolated the novel antifungal metabolite KB425796-A produced by the recently isolated bacterium Paenibacillus sp. 530603. On the basis of morphological changes of Aspergillus fumigatus induced by KB425796-A in combination with micafungin, we developed a highly sensitive screening method for the specific detection of KB425796-A congeners. Using this method, we isolated ten congeners of KB425796-A, named KB425796-B, -C, -D, -E, -F, -G, -H, -I, -J and -K, which exhibited diverse antifungal potencies against A. fumigatus. One of the most potent congeners, KB425796-C, had antifungal activities against several micafungin-resistant infectious fungi. KB425796-C can be a potential drug candidate for treating micafungin-resistant fungal infections.
Similar content being viewed by others
Introduction
The discovery and characterization of natural congeners is a promising approach for understanding the relationship between the chemical structure and biological function of a target compound. Developing an assay system based on the specific biological activity of the parental compound is a key to identifying novel congeners from the fermentation broth.
In a previous paper, we reported the isolation of the novel antifungal compound KB425796-A from the culture supernatant of Paenibacillus sp. 530603. Moreover, in the course of isolation procedures of KB425796-A, we found many minor peaks in the HPLC analysis of side fractions. We also showed that KB425796-A has unique in vitro activity against A. fumigatus, which was characterized by the swelling and bulging of hyphae. On the basis of our screening experience for antifungal natural products, we concluded that the morphological changes to A. fumigatus hyphae induced by KB425796-A were similar to those caused by nikkomycins.
Nikkomycins are nucleoside peptides that function as competitive inhibitors of chitin synthase in the fungal cell wall. Notably, nikkomycin Z and the echinocandin-like lipopeptide micafungin act synergistically against A. fumigatus, both in vitro and in vivo.1, 2 This observation led us to evaluate the antifungal activity of KB425796-A against A. fumigatus in combination with micafungin. We also expected the synergistic activity would allow us to detect minor, but potent, congeners of KB425796-A from the culture broth of strain 530603. In the present paper, we obtained fractions from the fermentation broth of strain 530603 and assayed the antifungal activity of each fraction in combination with micafungin. Our screening led to the identification of ten new compounds, KB425796-B, -C, -D, -E, -F, -G, -H, -I, -J and -K, whose physicochemical properties and antifungal activities were then evaluated and compared.
Results
Morphological changes of A. fumigatus induced by KB425796-A
A. fumigatus was treated with the indicated concentrations of micafungin and KB425796-A, either alone or in combination (Figure 1). Morphological examination of A. fumigatus treated with micafungin alone showed truncated, branched and shortened hyphae, whereas KB425796-A induced the swelling and bulging of hyphae. However, when these drugs were used in combination, A. fumigatus hyphae exhibited numerous spherical dilatations. Such spherical morphology could also be observed in hyphae treated with the fractions adjacent to the active fractions in each purification step of KB425796-A. Thus, we attempted to isolate the active substances from these secondary fractions.
Changes in the hyphal structure of A. fumigatus treated with KB425796-A and micafungin alone and in combination for 17 h. Light microscopy images of (a) Control cells, and cells treated with (b) micafungin 0.5 μg ml−1, (c) KB425796-A 3.1 μg ml−1, (d) micafungin 0.5 μg ml−1+KB425796-A 0.8 μg ml−1, (e) Nikkomycin X 6.3 μg ml−1 and (f) micafungin 0.5 μg ml−1+Nikkomycin X 6.3 μg ml−1 are shown. Scale bar, 100 μm.
Isolation and physicochemical properties of KB425796-B to -K
To isolate minor congeners of KB425796-A from the fermentation broth of strain 530603, the broth was extracted with acetone. The extract was further purified using the procedure outlined in Figure 2. All obtained fractions were subjected to an antifungal assay in combination with micafungin, and the fractions that induced distinctive morphological change of hyphae, as described in detail above, were selected as active fractions. A total of ten KB425796-A congeners were isolated from the active fractions (Figure 3).
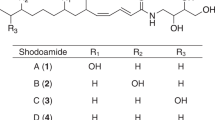
The physicochemical properties of KB425796-B to -K are summarized in Table 1. All congeners were soluble in methanol and dimethyl sulfoxide, sparingly soluble in chloroform and acetone, and insoluble in water and ethyl acetate. The congeners displayed positive color reaction to iodine vapor, ceric sulfate and ninhydrin, but displayed negative reactions against Molish, Dragendorff and FeCl3. The UV absorption maxima of KB425796-B to -K were observed at 280 and 290 nm. Absorptions at 1650 and 1540 cm−1 in the IR spectra indicated the presence of peptide bonds. The HR-ESI-MS measurements of all the ten congeners produced doubly protonated molecules, [M+2H]2+, but singly protonated molecules, [M+H]+, also appeared as a weak peak.
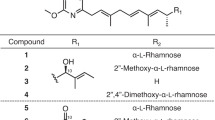
The exact molecular mass of KB425796-C was found to be 1646.9061 Da ([M+H]+), corresponding to the molecular formula C81H119N19O18 (theoretical: [M+H]+ 1646.9059 Da), which was C2H4 more than the molecular formula of KB425796-A. The 1H NMR spectrum of KB425796-C was quite similar to that of KB425796-A, with the exception of the intensity of the integral area (δH 1.30–1.20). In addition, comparison of 13C NMR data revealed that KB425796-C possessed two extra methylene signals (δC 30.7 (t) and 30.6 (t)). 2-methylpropyl and 3-hydroxybutyryl units were evident from the COSY, HSQC and HMBC analyses. Together, these data indicated that the 3-hydroxy-13-methylmyristoyl moiety in KB425796-A was substituted with a 3-hydroxy-15-methylpalmitoyl moiety in KB425796-C, as shown in Figure 3. With the proposed structure of KB425796-C in hand, a detailed amino-acid analysis of KB425796-C was performed.
Using conventional amino-acid hydrolysis conditions (6 M HCl, 110 °C, 18 h), tryptophan (Trp) and 5-hydroxytryptophan (HOTrp) present in the samples were destroyed. To detect acid-labile amino acids, such as Trp, a modified amino-acid analysis method with 6 M HCl containing 4% thioglycolic acid as a hydrolysis solution at 110 °C was used. The acid hydrolysis of KB425796-C gave equimolar amounts of Asp, HOTrp, Trp, His, Pro, two equivalents (eq.) of Val and three eq of Gly. In addition, 1.7 eq of Orn and 0.3 eq of citrulline (Cit) were detected. Under the same acid hydrolysis conditions, we detected that authentic Cit was hydrolyzed to afford 0.7 eq of Orn, indicating that 1 mol each of Cit and Orn were present in KB425796-C. The results of the amino-acid analysis were entirely consistent with the proposed structure of KB425796-C.
The structures of KB425796-B, -D, -E, -F, -G, -H, -I, -J and -K were tentatively determined by comparison of the HR-ESI-MS spectra and 1H and 13C NMR spectra (data not shown) with those of KB425796-C. Full account of structure elucidation of KB425796-A congeners will be described in a separate paper.
Antifungal activities of KB425796-A to -K
The antifungal activities of KB425796-A to -K were evaluated by the broth-dilution method and are summarized in Table 2. Although none of the compounds exhibited antifungal activity against Candida albicans, all were active against A. fumigatus and induced several morphological changes, such as the swelling and bulging of hyphae. In addition, the compounds displayed synergistic activity with micafungin against A. fumigatus, as determined by the induction of distinctive globular changes to hyphae. When used as a single agent, KB425796-A to -K had minimum effective concentrations ranging 2.5–10 μg ml−1 and exhibited fungistatic activities (MIC >50 μg ml−1). In contrast, the activities of these compounds in combination with 0.05 μg ml−1 of micafungin were highly potent and fungicidal against A. fumigatus, though both of the KB425796-A congeners and micafungin were fungistatic as single agents. The MICs under this condition ranged from 0.16 to 2.5 μg ml−1 and were lowest for KB425796-B, -C, -E, -F and -G (0.16 μg ml−1). These congeners, with the exception of KB425796-G, also exhibited the lowest minimum effective concentrations (2.5 μg ml−1) among all congeners. Interestingly, the compounds with the highest potency had C13-C14 fatty acid side chains, whereas those with the lowest potency had C12 side chains.
As KB425796-C was one of the most potent congeners against A. fumigatus and had the highest productivity, we evaluated the in vitro antifungal spectrum of KB425796-C and other reference antifungal agents against various yeast-like and filamentous infectious fungi (Table 3). KB425796-C exhibited potent antifungal activity against a variety of fungal species, with particularly high efficiency against Trichosporon asahii, which is resistant to azoles and candins, but it was relatively inactive against C. albicans. Microscopic observation of two filamentous fungi, Aspergillus fumigatus and Rhizopus oryzae, after treatment with KB425796-C revealed the swelling and bulging of hyphae. These morphological changes were similar to those observed in the hyphae treated with nikkomycin X, but differed from the changes induced by treatment with micafungin (Figure 1).
Discussion
In this study, we found that the combined treatment of A. fumigatus hyphae with micafungin and nikkomycin X induced globular structures such as spheroplasts (Figure 1). This finding corresponded to the reports that micafungin and nikkomycin Z act synergistically both in vitro and in vivo against A. fumigatus.1, 2
As the morphological changes induced by KB425796-A were similar to those of nikkomycins, here we evaluated the antifungal effects of KB425796-A against A. fumigatus alone and in combination with micafungin. Micafungin induced the formation of truncated, branched and shortened hyphae, whereas KB425796-A induced the swelling and bulging of hyphae. When the compounds were used in combination, spherical dilatations were observed microscopically. The minimum concentration of KB425796-A that had an effect on A. fumigatus hyphae was lower in combination with micafungin than as a single agent. This property allowed us to develop a biological assay for identifying minor derivatives of KB425796-A with high specificity and sensitivity.
In the present study, we purified ten new congeners of KB425796-A from the culture supernatant of strain 530603 using preparative HPLC and aminopropyl silica gel chromatography. We have also succeeded in isolating another microorganism that produces congeners of KB425796-A using the antifungal assay method developed here (data not shown). Thus, the micafungin combination assay technique may be a powerful screening tool for discovering antifungal compounds, such as the KB425796-A congeners identified here, but also novel derivatives of nikkomycin.
In the course of the structural analysis of the isolated minor congeners of KB425796-A, 2D NMR analysis revealed that these compounds have the same scaffold as KB425796-A. Similarity in the physicochemical properties of these compounds also supports this finding. Although none of the compounds had antifungal activity against C. albicans, all of them induced the swelling and bulging of A. fumigatus hyphae, and displayed a synergistic effect when used in combination with micafungin. These conserved features of the identified congeners suggest that they also share a common mechanism of action in addition to the similarity of their chemical structures. The amino-acid sequence in the cyclic peptide nucleus of KB425796-A congeners is likely important for their antifungal activity because two known 40-membered macrocyclic lipopeptidelactones, WAP-82943 and FR901469,4, 5 do not display such synergistic activity with micafungin. Notably, however, differences in the level of antifungal activity against A. fumigatus were detected between the ten congeners, with KB425796-B, -C, -E and -F having higher potency than KB425796-A. This observation suggests that derivatives with long fatty-acid side chains tend to have more potent antifungal activity.
Analysis of the antifungal spectrum activity of KB425796-C revealed this compound has potent activity against a variety of fungal species, particularly T. asahii. As members of the genus Trichosporon are generally less susceptible to amphotericin B, micafungin and fluconazole6 than C. albicans and A. fumigatus, the treatment for systemic trichosporonosis remains inadequate. Our present findings suggest that KB425796-C is a potential drug candidate for treating systemic trichosporonosis. Similar to the case of KB425796-A, microscopic observation of the filamentous fungi A. fumigatus and R. oryzae after treatment with KB425796-C revealed the swelling and bulging hyphae, which is similar to the morphological changes observed in the hyphae treated with nikkomycin X, but differed from those induced by micafungin. This finding suggests that the mode of action of KB425796-C is based on the inhibition of cell wall components.7, 8 We plan to evaluate the in vivo activities of KB425796-C against T. asahii and A. fumigatus and will describe the findings in a following paper.
Methods
HPLC analysis
The detection of KB425796-A congeners in fermentation broth and column fractions during purification was performed by HPLC using a reverse phase column (L-column ODS, 250 mmφ × 4.6 mm I.D.; Chemical Evaluation and Research Institute, Japan). An aqueous acetonitrile solution (42%) containing 0.1% TFA was used as the mobile phase at a flow rate of 1.0 ml min−1. The detection wavelength was set at 210 nm.
Antimicrobial activity
C. albicans FP633, T. asahii FP2044, R. oryzae FP1988, Fusarium solani FP1930, Pseudallescheria boydii FP1987, and Trychophyton mentagrophytes FP2103, which are clinical isolates deposited in our laboratory, were incubated statically in yeast-maltose (YM) agar broth for 24 h at 30 °C. Cryptococcus neoformans YC203 (deposited in our laboratory) was cultured in YM broth medium for 20 h at 30 °C with shaking at 250 r.p.m. A cell suspension was prepared by washing the cultured cells once with sterile saline. Aspergillus fumigatus FP1305 (deposited in our laboratory) was cultured on a potato dextrose agar (PDA) slant for 4 days, and spores were then harvested in sterile saline and collected by filtering through gauze. The antimicrobial activity was measured by the micro-broth dilution method in 96-well culture plates using RPMI 1640 medium (Invitrogen Japan, Tokyo, Japan) supplemented with L-glutamine, but without sodium bicarbonate, and buffered to pH 7.0 with 0.165 M MOPS. Yeast nitrogen base-glucose (YNBD) medium was used for measuring antimicrobial activity against C. neoformans. For the measurements, the test microorganism was inoculated into each at a final concentration of 1 × 105 c.f.u. perwell. The plates were incubated for 20 h (C. albicans FP633, T. asahii FP2044, R. oryzae FP1988, P. boydii FP1987, F. solani FP1930, and A. fumigatus FP1305) or 48 h (C. neoformans YC203 and T. mentagrophytes FP2103) at 37 °C. Two end points were determined by microscopic observation: minimum effective concentration, which was the lowest concentration causing a substantial reduction in fungal growth, and MIC, which was the concentration needed completely inhibited growth.
Isolation and purification of KB425796-B, -C, -D, -E, -F, -G, -H, -I, -J and -K
The fermentation of Paenibacillus sp. 530603 was performed as previously described9. The supernatant of the fermentation broth was extracted with acetone and subjected to HP-20 (Mitsubishi Chemical Co., Ltd., Tokyo, Japan) and ODS column chromatography (Daisogel SP-120-ODS-B, 15/30 μm; Daiso Co., Ltd., Osaka, Japan).9 Each fraction obtained from the ODS column chromatography was analyzed by HPLC and used for the antifungal assay. Active fractions containing the same substances, as determined by HPLC, were pooled and concentrated in vacuo to yield a mixture of KB425796-A congeners. This crude preparation of compounds was dissolved in 2 ml methanol and purified by preparative HPLC using an L-column (250 × 20 mm I.D.; Chemical Evaluation and Research Institute, Japan) with 42% aqueous acetonitrile containing 0.1% formic acid as elution solvent. Active fractions were concentrated in vacuo to yield a white powder, which was dissolved in a minimum amount of methanol and purified by aminopropyl silicagel chromatography (75/150 μm; Tokyo Chemical Industry Co., Ltd., Tokyo, Japan) using methanol:chloroform (1:1) as an elution solvent. Active fractions were analyzed using HPLC and TLC under the conditions described above and in Table 1. The fractions including each congener were evaporated and congeners KB425796-B to -K were obtained as white powder.
Amino-acid analysis
Amino-acid analysis was performed using the following procedure. KB425796-C, citrulline, ornithine and 5-hydroxytryptophan were subjected to hydrolysis in 6 M HCl containing 4% thioglycolic acid for 22 h at 110 °C.10 The samples were then placed in a freeze-dryer to remove HCl under reduced pressure. The amino acids yielded by hydrolysis were analyzed using a high performance amino-acid analyzer (L-8800A; Hitachi High-Technologies Co., Tokyo, Japan).
References
Chiou, C. C., Mavrogiorgos, N., Tillem, E., Hector, R. & Walsh, T. J. Synergy, pharmacodynamics, and time-sequenced ultrastructural changes of the interaction between nikkomycin Z and the echinocandin FK463 against Aspergillus fumigatus. Antimicrob. Agents Chemother. 45, 3310–3321 (2001).
Clemons, K. V. & Stevens, D. A. Efficacy of micafungin alone or in combination against experimental pulmonary aspergillosis. Med. Mycol. 44, 69–73 (2006).
Kato, A. et al. A new anti-MRSA antibiotic complex, WAP-8294A. I. Taxonomy, isolation and biological activities. J. Antibiot. 51, 929–935 (1998).
Fujie, A. et al. FR901469, a novel antifungal antibiotic from an unidentified fungus No.11243. I. Taxonomy, fermentation, isolation, physico-chemical properties and biological properties. J. Antibiot. 53, 912–919 (2000).
Fujie, A. et al. FR901469, a novel antifungal antibiotic from an unidentified fungus No.11243. II. In vitro and in vivo activities. J. Antibiot. 53, 920–927 (2000).
Tawara, S. et al. In vitro activities of a new lipopeptide antifungal agent, FK463, against a variety of clinically important fungi. Antimicrob. Agents Chemother. 44, 57–62 (2000).
Debono, M. & Gordee, R. S. Antibiotics that inhibit fungal cell wall development. Annu. Rev. Microbiol. 48, 471–497 (1994).
Liu, J. & Balasubramanian, M. K. 1,3-beta-Glucan synthase: a useful target for antifungal drugs. Curr. Drug Targets Infect. Disord. 1, 159–169 (2001).
Kai, H. et al. KB425796-A, a novel antifungal antibiotic produced by Paenibacillus sp. 530603. J. Antibiot. 66, 465–471 (2013).
Matsubara, H. & Sasaki, R. M. High recovery of tryptophan from acid hydrolysates of proteins. Biochem. Biophys. Res. Commun. 35, 175–181 (1969).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kai, H., Yamashita, M., Takase, S. et al. Identification of ten KB425796-A congeners from Paenibacillus sp. 530603 using an antifungal assay against Aspergillus fumigatus in combination with micafungin. J Antibiot 66, 473–478 (2013). https://doi.org/10.1038/ja.2013.64
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2013.64