Abstract
Two new diterpenes, libertellenone G(1) and libertellenone H(2) were isolated from the fungus Eutypella sp. D-1 isolated from the soil of high latitude of Arctic, together with two known pimarane diterpenes (3–4). The structures of 1 and 2 were elucidated from spectroscopic data (nuclear magnetic resonance, mass spectrometry and infrared). These compounds were evaluated for cytotoxic activity against seven human tumor cell lines. Compound 2 showed a range of cytotoxicity between 3.31 and 44.1 μM. Compound 1 exhibited antibacterial activity against Escherichia coli, Bacillus subtilis and Staphylococcus aureus.
Similar content being viewed by others
Introduction
Polar regions are remote and challenging areas on the Earth because of the severe conditions of low temperature, low water availability, frequent freeze-thaw cycles, strong winds and so on. Microorganisms are the main dominator of the polar region.1, 2 Microorganisms, especially fungi, have proven to be an attractive source of new bioactive secondary metabolites.3, 4, 5 Recently, we isolated a fungus strain, Eutypella sp. D-1 from the soil of high latitude of Arctic.
So far, only a small number of studies about the secondary metabolites of fungus Eutypella sp. have been reported. The secondary metabolites of this genus were polyketides such as γ-lactones, benzopyran derivatives and cytosporin-related compounds, terpenoids such as ent-eudesmane sesquiterpenes, pimarane diterpenes, nitrogenous compounds such as cytochalasin derivatives and cyclic dipeptides.6, 7, 8, 9, 10
Results and discussion
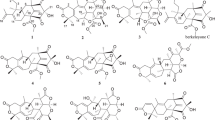
In the course of our studies on bioactive components produced by polar-derived fungi isolated from Arctic, we found that the ethanol (EtOAc) extract of the culture broth from a fungus, Eutypella sp. D-1, isolated from the soil of high latitude of Arctic, showed cytotoxicity against a human breast cancer cell line MCF-7. Bioassay-guided separation from the EtOAc extract led to the isolation of new libertellenone G(1), libertellenone H(2) (Figure 1) and two known compounds libertellenone A(3) and libertellenone C(4).10 All isolated compounds were tested for cytotoxicity against seven human tumor cell lines. Compound 2 showed a range of cytotoxicity between 3.31 and 44.1 μM. Herein we report the isolation, structural determination and biological activity of these four compounds.
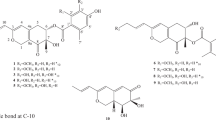
Libertellenone G (1) was isolated as yellow oil, which was analyzed for the molecular formula C20H26O3 by high-resolution electrospray ionization (ESI) mass spectrometry(MS) m/z (M+H)+ 315.1963. On the basis of detailed analysis of nuclear magnetic resonance (NMR) data, it revealed that libertellenone G contains 20 carbons including one carbonyl (δC 182.4), 7 quarternary carbons, 3 methines, 6 methylenes and 3 methyls. Further, the 1H NMR spectrum showed five olefinic protons at H-15 (δH 5.71), H-16a (δH 4.94), H-16b (δH 4.85), H-1 (δH 5.91) and H-2 (δH 6.00). The heteronuclear multiple bond correlation (Figure 2) correlations from H-15 to C-12 and C-17, H-16 to C-14, C-15 and C-16, H-1 to C-3, C-5, C-9 and C-10, and H-2 to C-10 suggested the presence of a pimarane-type diterpene. The spectroscopic data of 1 were similar to those of libertellenone B,11 except for the double bond of C-9 replacing hydroxyl group. The heteronuclear multiple bond correlation spectrum indicated that H-11 (2.49, 2.39 p.p.m., m), H-12 (2.2, 1.51 p.p.m., m) and H-20 (1.4 p.p.m., s) coupled to C-8 (127.2 p.p.m., s), H-1(5.91 p.p.m., ddd), H-14 (1.7, 2.52 p.p.m., m) and H-20 (1.4 p.p.m., s) coupled to C-9 (162.9, s), H-12 (2.2, 1.51 p.p.m., m), H-16 (4.94, 4.85 p.p.m., dd) and H-17 (1.05 p.p.m., s) coupled to C-14 (33.6, t). Complete assignment of protons and carbons in 1 is shown in Table 1.
Libertellenone H (2) was obtained as yellow oil, and the molecular formula was deduced to be C26H34O7 by high-resolution ESI-MS ([m/z (M+H)+ 458.2294]). The 1H and 13C NMR spectral data for 2 were similar to those of 1, which indicated that compound 2 was a pimarane diterpene derivative. Compared with the literature, compound 2 was more similar to libertellenone D,11 exhibiting a characteristic chemical shift for the H-20 protons (0.5, 1.11, m). Besides, compound 2 exhibited two more carbonyl carbon signals at C-21 (δC 169.9) and C-23 (δC 176.9). Further analysis of heteronuclear multiple bond correlation NMR data (Figure 2) indicated that H-18 (4.38, 4.74 p.p.m., d), H-24 (2.5, p.p.m., m), H-25 (1.12 p.p.m., d) and H-26 (1.14 p.p.m., d) were coupled to C-23 (176.9 p.p.m., qC). In addition, H-3 (4.94 p.p.m., dd) and H-22 (2.04 p.p.m., s) coupled to C-21 (169.9 p.p.m., qC). It was further deduced that compound 2 included two ester bonds. Interpretation of additional heteronuclear multiple bond correlation correlations established the full planar structure of this diterpenoid.
The relative stereochemistry of compounds 1 and 2 were assigned by analysis of nuclear Overhauser effect spectroscopy and by 1H NMR coupling constant data. In compound 1, the CH3-20 showed nuclear Overhauser effect correlations with CH3-19 and CH3-17. In compound 2, the CH2-20 showed NOE correlations with CH3-19 and CH3-17. These correlations demonstrated that C-17, C-19 and C-20 are in axial configurations on the top face of the molecule. (Figure 3) Thus, compounds 1 and 2 were elucidated as new pimarane diterpene derivatives.
Two known compounds libertellenone A (3) and libertellenone C (4) were identified on the basis of their spectroscopic profiles (NMR, ultraviolet, infrared (IR) and MS) and comparison with published data.11
Compounds 1–4 were tested for their in vitro cytotoxic activity against a series of tumor cell lines by the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] method.12 Compound 2 showed slight cytotoxicity toward most cell lines, with half-maximal inhibitory concentration values ranging from 3.31 to 44.1 μM (Table 2). Interestingly, despite its small structural differences between these four compounds, the biological activity showed big differences. This suggests that the cyclopropane ring in 2 appears to be an important structural feature associated with the biological activity of this compound, which has been shown in the earlier paper.11
Besides, Compounds 1–4 were tested for their antibacterial activity. Compounds were tested at 50 μg placed on 6 mm paper disks, compound 1 showed moderate antibacterial activities against Escherichia coli, Bacillus subtilis and Staphylococcus aureus (Table 3).
In this study, Libertellenone G (1) and Libertellenone H (2) were elucidated as new pimarane diterpene derivatives, which exhibited good cytotoxicity activity and antibacterial activity. The cyclopropane ring in 2 appears to be an important structural feature associated with the biological activity of libertellenone family.
Experimental Procedure
General
IR spectra were recorded on a Bruker Vector-22 spectrometer (Bruker Corporation, Billerica, MA, USA) with KBr pellet. NMR spectra were measured on a Bruker DRX-600 spectrometer at 600 MHz for 1H NMR and 150 MHz for 13C NMR with tetramethylsilane (TMS) as the internal standard. ESI-MS were recorded on a Varian Q-TOF micro mass spectrometer (Varian Corporation, Palo Alto, CA, USA) and ultraviolet data were obtained with a Shimadzu UV-265 (Shimadzu Corporation, Tokyo, Japan). High-performance liquid chromatography was performed on a high-pressure gradient equipped with Waters 510 high-performance liquid chromatography pump and Waters 2996 Photodiode Array Detector (Waters Corporation, Milford, MA, USA). Mid-pressure liquid chromatography was equipped with Pump Module C-605, Pump manager C-615, Fraction Collector C-660 and UV-Photomaker C-635 (Buchi Corporation, Flawil, Switzerland). Column chromatography was performed on Sephadex LH-20 (Pharmacia Corporation, Piscataway, NJ, USA); thin layer chromatography analysis was run on HSGF254-precoated silica-gel plates (10–40 μm, Yantai Chemical Plant, Yantai, China). All other reagents were of analytical grade (Shanghai Chemical Plant, Shanghai, China).
Fungal strain
The fungus was isolated from the soil of London Island of Kongsfjorden of Ny-Ålesund District (altitude of 100 m) of Arctic. It was isolated on potato dextrose agar medium with incubation at 20 °C. Because of its 18S ribosome DNA (rDNA) (GenBank Accession number FJ430580), the strain can be assigned to the genus Eutypella sp. A voucher specimen (number 0605020) was deposited in the potato dextrose agar medium at the Second Military Medical University, Xiangyin Road 800, 200433, Shanghai, China.
Culture condition
Eutypella sp. D-1 was cultured in potato dextrose broth (potato 1%, glucose 2%, Hangzhou Microbial Reagent Co., LTD, Hangzhou, China). The fungus was maintained on potato dextrose agar medium at 20 °C for 7 days, and then three pieces (0.5 × 0.5 cm2) of mycelial agar plugs were inoculated into 60 × 250 ml2 Erlenmeyer flasks, each containing 70 ml potato dextrose broth. After 5 days of incubation at 20 °C on a rotary shaker at 180 r.p.m., 70 ml seed cultures were transferred into a total of 80 flasks (2 l) containing 700 ml potato dextrose broth. The liquid cultivation that followed was kept for 9 days at 20 °C and 180 r.p.m. on a rotary shaker.
Extraction and isolation
The culture (60 l) was centrifuged to give the broth and mycelia. The broth was exhaustively extracted with EtOAc (60 l × 3) three times, and then the EtOAc layers were combined and evaporated under reduced pressure at a temperature not exceeding 40 °C to yield a dark brown gum (6.34 g). The crude EtOAc extracts were subjected to Sephadex LH-20 (10 × 100 cm2) with methanol (MeOH; 5 l) eluting, to afford six fractions: A (500–800 ml, 1.22 g), B (800–2000 ml, 1.88 g), C (2000–3000 ml, 1.34 g), D (3000–3500 ml, 0.78 g), E (3500–4500 ml, 0.52 g) and F (4500–5000 ml, 0.6 g). The fraction C (1.34 g) was rechromatographed on middle-pressure liquid chromatography with the gradient CH3OH/H2O (5–100%) to give five fractions, C-1 (5–25%, 0.188 g), C-2 (25–40%, 0.253 g), C-3 (40–55%, 0.305 g), C-4 (55–75%, 0.225 g) and C-5 (75–100%, 0.228 g). Then fraction C-3 (0.305 g) was rechromatographed on middle-pressure liquid chromatography with 60% CH3OH/H2O to give compound 1 (between 800 and 1200 ml, 7 mg), fraction C-4 (0.225 g) was rechromatographed on middle-pressure liquid chromatography with 60% CH3OH/H2O (between 1400 and 1800 ml) and preparative thin layer chromatography on a silica-gel plate with chloroform-CH3OH (20:1) to give compound 2 (8.7 mg).
The purity of the isolated compounds (compound 1–4) was analyzed by high-performance liquid chromatography using a C18 column and ultraviolet detection at 218 nm. The compounds were eluted using a gradient mobile phase consisting of (A) acetonitrile and (B) water at a flow rate of 1.0 ml min−1. The elution program involved a linear gradient from 0 to 100% of solvent A to B within 0–30 min. The purities of isolated compounds 1, 2, 3 and 4 all exceeded 98%.
Libertellenone G (1): yellow amorphous powder, [α]25D=78.4 (c 0.2, MeOH) (see Supplementary Figure 2), IR ν (KBr) cm−1: 3402, 2913, 1638, 1328, 1035, ultraviolet λmax (MeOH) (log ɛ) 204 (3.98), 260 (3.82), 310 (3.52) (see Supplementary Figure 3); high-resolution MS m/z (M+H)+ 315.1963 (calcd for C20H26O3, 315.1952) (see Supplementary Figure 1), 1H and 13C NMR data, see Table 2 (see Supplementary Figures 4 and 5).
Libertellenone H (2): yellow amorphous powder, [α]25D=−70.1 (c 0.2, MeOH) (see Supplementary Figure 11), IR ν (KBr) cm−1: 3392, 2956, 1738, 1644, 1368, 1288, 1035, ultraviolet λmax (MeOH) (log ɛ) 202 (4.01), 261(3.77), 330(3.73) (see Supplementary Figure 12); high-resolution MS m/z (M+H)+ 459.2378 (calcd for C26H35O7, 459.2362) (see Supplementary Figure 10), 1H and 13C NMR data, see Table 2 (see Supplementary Figures 13 and 14).
Bioassay
All isolates obtained in this study were evaluated for their cytotoxic activity using established methods.12 Adriamycin, 5-fluorouracil, paclitaxel (Sigma-Aldrich, St Louis, MO, USA; 97% pure) were used as positive control. The purities of isolated compounds 1, 2, 3 and 4 were found to be 99.4%, 98.8%, 98.6% and 98.5%, respectively.
Individual compound (1–4, 50 μg) were loaded on filter paper to evaluate their antibacterial activity using established methods.13 Ampicillin (Sigma-Aldrich; 98% pure) were used as positive control. The purities of isolated compounds 1, 2, 3 and 4 were found to be 99.4%, 98.8%, 98.6% and 98.5%, respectively.
References
Friedmann, E. I. Antarctic microbiology, Wiley-Liss: New York, NY, USA, (1993).
Liu, J. T. et al. Bioactive natural products from the antarctic and arctic organisms. Mini Rev. Med. Chem. 13, 617–626 (2013).
Faulkner, D. J. Marine natural products. Nat. Prod. Rep. 19, 1–48 (2002).
Blunt, J.W., Copp, B.R., Munro, M.H.G., Northcote, P.T. & Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 29, 144–222 (2012).
Hill, R.A. Marine natural products. Annu. Rep. Prog. Chem. 108, 131–146 (2012).
Ciavatta, M. L. et al. Cytosporin-related compounds from the marine-derived fungus Eutypella scoparia. Tetrahedron 64, 5365–5369 (2008).
Isaka, M. et al. γ-Lactones and ent-eudesmane sesquiterpenes from the endophytic fungus Eutypella sp. BCC 13199. J. Nat. Prod. 72, 1720–1722 (2009).
Pongcharoen, W., Rukachaisirikul, V., Phongpaichit, S., Rungjindamai, N. & Sakayaroj, J. Pimarane diterpene and cytochalasin derivatives from the endophytic fungus Eutypella scoparia PSU-D44. J. Nat. Prod. 69, 856–858 (2006).
Isaka, M., Palasarn, S., Prathumpai, W. & Laksancharoen, P. Pimarane diterpene from the endophytic fungus Eutypella sp. BCC 13199. Chem. Pharm. Bull. 59, 1157–1159 (2011).
Sun, L. et al. new oxygenated pimarane diterpenes from the marine sediment-derived fungus Eutypella scoparia FS26. Mar. Drugs 10, 539–550 (2012).
Oh, D. Ch., Jensen, P. R., Kauffman, C. A. & Fenical, W Libertellenones A-D: induction of cytotoxic diterpenoid biosynthesis by marine microbial competition. Bioorg. Med. Chem. 13, 5267–5273 (2005).
Liu, R. et al. 10-phenyl-[12]-cytochalasins Z7, Z8 and Z9 from the marine-derived fungus Spicaria elegans. J. Nat. Prod. 69, 871–875 (2006).
Gabhainn, S. N. et al. The precision and robustness of published protocols for disc diffusion assays of antimicrobial agent susceptibility: an inter-laboratory study. Aquaculture 240, 1–18 (2004).
Acknowledgements
We are grateful to Professor Bo Chen (Polar Research Institute of China) to supply the Arctic fungus Eutypella sp. D-1. The work was funded by National Hi-tech R&D Program of China (863 Program) (SS2012AA09160703), National Natural Science Foundation of China (NSFC) (41306197).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary Information accompanies the paper on The Journal of Antibiotics website
Supplementary information
Rights and permissions
About this article
Cite this article
Lu, XL., Liu, JT., Liu, XY. et al. Pimarane diterpenes from the Arctic fungus Eutypella sp. D-1. J Antibiot 67, 171–174 (2014). https://doi.org/10.1038/ja.2013.104
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2013.104
Keywords
This article is cited by
-
Anti-inflammatory effects of TP1 in LPS-induced Raw264.7 macrophages
Applied Biological Chemistry (2024)
-
Antimicrobial compounds from marine fungi
Phytochemistry Reviews (2021)
-
Stimulatory effect of ethanol on libertellenone H biosynthesis by Arctic fungus Eutypella sp. D-1
Bioprocess and Biosystems Engineering (2016)