Abstract
We investigated whether thrombin-cleaved osteopontin N-terminal is useful as a blood biomarker of acute atherothrombotic ischemic stroke. Acute ischemic stroke patients were prospectively evaluated with brain magnetic resonance imaging and cardiac evaluations for etiological diagnosis according to the Trial of Org 10172 in Acute Stroke Treatment classification. They were divided into the atherothrombotic and non-atherothrombotic groups. Thrombin-cleaved osteopontin N-terminal, osteopontin, matrix metalloproteinase-9, S100B, C-reactive protein and D-dimer levels were measured from blood samples collected at admission. After excluding patients who met the exclusion criteria or had stroke of other/undetermined etiology, 60 of the 100 patients initially enrolled were included in the final analysis. The ischemic stroke subtypes were atherothrombotic (n=28, 46.7%), cardioembolic (n=19, 31.7%) and lacunar (n=13, 21.7%). Thrombin-cleaved osteopontin N-terminal and matrix metalloproteinase-9 levels were significantly higher in the atherothrombotic than in the non-atherothrombotic group (median (interquartile range): 5.83 (0.0–8.6 ) vs. 0.0 (0.0–3.3) pmol l−1, P=0.03 and 544 (322–749 ) vs. 343 (254–485) ng ml−1, P=0.01, respectively). After adjustment for the prevalence of hypertension, diabetes and dyslipidemia, thrombin-cleaved osteopontin N-terminal levels of >5.47 pmol l−1 (odds ratio, 16.81; 95% confidence interval, 3.53–80.10) and matrix metalloproteinase-9 levels of >605.5 ng ml−1 (6.59; 1.77–24.60) were identified as independent predictors of atherothrombosis. Within 3 h from stroke onset, only thrombin-cleaved osteopontin N-terminal independently predicted atherothrombosis and thus may add valuable, time-sensitive diagnostic information in the early evaluation of ischemic stroke, especially the atherothrombotic subtype.
Similar content being viewed by others
Introduction
Although stroke mortality has gradually decreased in Japan, primarily owing to the control of hypertension,1 stroke still causes long-term disability and death in 121 000 people per year.2 Recently, ischemic stroke among Japanese patients have changed from the small- to the large-vessel subtype, including the atherothrombotic or cardioembolic subtypes.3, 4 These subtypes showed different arteriosclerotic indicators.5 The prognoses and responses to endovascular treatment differ among these subtypes. The atherothrombotic subtype is more highly associated with early recurrence than the cardioembolic or lacunar subtype.6 After thrombolysis therapy, endovascular treatment leads to greater recanalization in the atherothrombotic subtype than in the cardioembolic subtype.7, 8 According to reports, the stroke subtype should be diagnosed correctly in the acute phase. The most effective diagnostic tool for stroke is neuroimaging. Diffusion-weighted imaging with magnetic resonance (MR) imaging (MRI) is sensitive for detecting ischemia. Furthermore, identification of the stroke subtypes still largely relies on a combination of other tests, including the use of MR angiography, carotid ultrasonography and transcranial Doppler imaging. Not only structural imaging tests but also a blood biomarker that would indicate the atherothrombotic subtype in the acute phase is warranted. Several blood markers have been reported as diagnostic biomarkers of acute ischemic stroke, such as matrix metalloproteinase (MMP)-9, S100B and D-dimer.9 However, whether these can discriminate among pathologic subtypes is unclear.
Osteopontin (OPN) is an extracellular matrix protein that plays a key role in inflammation and malignant tumors.10 In atherosclerosis, the plasma OPN level has a positive relationship with local11 and systemic atherosclerosis burden.12 When OPN is cleaved by thrombin, it transforms into two types, the thrombin-cleaved OPN N-terminal (trOPN-N) and C-terminal (trOPN-C) fragments. We previously reported the presence of trOPN-N in the carotid artery in highly inflamed atherosclerosis; after its mechanical destruction by carotid artery stenting, the plasma trOPN-N level quickly increased.13 Considering that the pathophysiological mechanism of atherothrombotic ischemic stroke is related to plaque instability and the resulting thrombogenic condition,14 trOPN-N is a potential biomarker to explain atherosclerotic and thrombotic states.
In this study, we evaluated the utility of trOPN-N, OPN, MMP-9, C-reactive protein, S100B and D-dimer as diagnostic biomarkers of the atherothrombotic subtype of ischemic stroke. Furthermore, we clarified the usefulness of these biomarkers in the acute setting.
Methods
Study participants, ethical considerations, consent and permissions
Patients were enrolled prospectively from July 2011 to March 2014. This study was designed as a pilot trial to assist in the development of a larger clinical trial. Participants were recruited from Ehime University Hospital, Matsuyama Saiseikai Hospital, Imabari Saiseikai Hospital and Okujima Hospital in Ehime, Japan. This study was performed in accordance with the Declaration of Helsinki. On behalf of all the participating facilities, approval from the institutional review board of Ehime University Hospital was obtained before study initiation (No. 1205005). In addition, the trial was registered in the University Hospital Medical Information Network (UMIN) clinical trial registry (No. 20183). Each participant was provided a detailed explanation of this study, and all patients or a relative signed a written statement of informed consent for participation and publication of the patients’ data. Acute ischemic stroke patients admitted within the first 24 h after symptom onset were enrolled. Patients with intracranial hemorrhage, known inflammatory or malignant disease, abnormal coagulant disease, or contraindication to MRI; those treated with recombinant tissue plasminogen activator before blood sampling; or those who had undergone cardiovascular operation within the previous 6 months were excluded from the final analysis.
Diagnosis of the ischemic stroke subtypes
Acute ischemic stroke was diagnosed by stroke neurologists and confirmed on MRI. The final diagnosis of the stroke subtype was rendered by the treating-site clinician, who was blinded to the biomarker results. Independent stroke experts reviewed all the clinical, imaging and conventional laboratory information gathered during admission.
The stroke etiological subtype was determined at admission based on the Trial of Org 101172 in Acute Stroke Treatment (TOAST) criteria as follows:15, 16 (1) large-vessel atherothrombotic; (2) cardioembolic; (3) small-vessel/lacunar; or (4) stroke of other determined etiologies or an undetermined etiology. Both the atherothrombotic and cardioembolic subtypes were classified based on an infarct size of >1.5 cm, as confirmed on MRI. The atherothrombotic subtype was defined as >50% stenosis of an appropriate intracranial or extracranial artery. Stenosis was calculated with MR angiography, computed tomography angiography or carotid ultrasonography according to the method used by the North American Symptomatic Carotid Endarterectomy Trial (NASCET). The cardioembolic subtype was determined to be middle- to high-risk without intracranial or extracranial artery stenosis. The lacunar subtype is characterized with traditional clinical neurological dysfunction and an infarct size of <1.5 cm as determined on MRI. The undetermined etiology subtype had two or more causes identified, and the ‘other determined etiologies’ subtype included perioperative stroke and arterial dissection. We excluded patients with undetermined etiology and ‘other determined etiologies’ subtypes from the final analysis because of their heterogeneous etiologies. Furthermore, we classified patients into atherothrombotic and non-atherothrombotic subtype groups to test our hypothesis.
Assessment of vascular risk factors
Patients were interviewed to determine the presence of diabetes mellitus, hypertension and/or dyslipidemia; smoking habits; and information regarding the medicines they took chronically prior to hospitalization. Hemoglobin A1c, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides and serum creatinine levels were measured. Diabetes mellitus was defined as the use of oral hypoglycemic agents or insulin, hemoglobin A1c levels of >6.5% or fasting blood glucose levels of >126 mg dl−1, or casual blood glucose levels of >200 mg dl−1. The systolic and diastolic blood pressure data used in the study were measured on admission for each patient. Hypertension was defined as the use of antihypertensive agents before hospitalization or its introduction during hospitalization. Dyslipidemia was defined as the use of antihyperlipidemic agents, low-density lipoprotein cholesterol levels of >140 mg dl−1 or high-density lipoprotein cholesterol levels of <40 mg dl−1, and/or triglyceride levels of >150 mg dl−1. A current smoker was defined as an individual with a history of smoking during the preceding 3 months, and a previous smoker was defined as an individual with a smoking history of >3 months prior to the ischemic attack. Electrocardiography, chest radiography, and transthoracic echocardiography were performed, additionally performing computed tomography angiography, carotid ultrasonography, and/or transesophageal echocardiography if indicated.
Determination of stroke size and severity
All the patients underwent MRI. The infarct volume was classified into three sizes by using MRI: (1) small: <1.5 cm, including multiple small infarcts; (2) large: >1 artery perfusion area; and (3) medium: intermediate between small and large. Stroke severity was assessed by using the National Institutes of Health Stroke Scale17 and the modified Rankin scale18 on admission and at discharge.
Biomarker assays
Blood samples were collected from the peripheral veins of all the patients at the emergency outpatient clinic before any treatment was administered. D-dimer and C-reactive protein levels were measured immediately after blood drawing. Plasma and serum were stored at −80 °C until analysis of OPN, trOPN-N, MMP-9, and S100B levels. Plasma was used for measurement of OPN and trOPN-N levels (code 27156 and 27258, respectively; Immuno Biological Laboratory, Fujioka, Japan); and serum, for MMP-9 (DMP900; R and D Systems, Minneapolis, MN, USA) and S100B (RD192090100R; Bio Vendor, Brno, Czech Republic) in enzyme-linked immunosorbent assay. Each sample was tested in duplicate.
Statistical analysis
All statistical analyses were performed with SPSS version 21 (SPSS, Chicago, IL, USA). Results of comparisons between two groups were analyzed by using the Student's t-test or Mann–Whitney U-test. Correlations between blood biomarkers and other clinical parameters were determined by using the Spearman rank-order test. A receiver-operating characteristic curve was used to obtain cutoff values with the Youden Index.19 Biomarkers associated with the atherothrombotic subtype in the univariate analysis were entered into a forward stepwise multivariate logistic regression model to identify independent predictors of the atherothrombotic subtype in comparison with those of the non-atherothrombotic subtype. A P-value of 0.05 was considered statistically significant.
Results
Participants
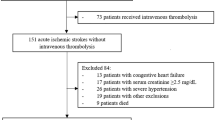
We obtained informed consent from 100 patients on admission and excluded 13 patients who met the exclusion criteria. We then excluded patients who had a stroke of undetermined etiology (n=27) and those who were receiving tissue plasminogen activator before blood sampling (n=1, included in the undetermined etiology). Finally, 60 patients were enrolled (Figure 1). The ischemic stroke subtypes identified were as follows: large artery atherosclerosis, n=28 (46.6%); cardioembolic, n=19 (31.7%); and lacunar, n=13 (21.7%). A descriptive analysis of the patients’ clinical backgrounds in both the atherothrombotic and non-atherothrombotic groups is summarized in Table 1. In brief, no significant differences in sex distribution, age, symptom duration, modified Rankin scale score, and National Institutes of Health Stroke Scale score on arrival and at discharge, and pre-hospitalization medications used were found between the groups. The atherothrombotic group had significantly more risk factors, including hypertension and diabetes, but less frequent atrial fibrillation.
Relationship between the biomarkers and the clinical characteristics
The intra-assay coefficients of variation for the assessed biomarkers were as follows: 13.7% for trOPN-N, 3.4% for OPN, 4.0% for S100B and 4.8% for MMP-9. As some trOPN-N values were negative after calibration with the baseline value, which contained a buffer with an antibody and a substrate but no blood sample, they were allocated a zero value. Table 2 shows the correlations of the biomarkers with the clinical backgrounds, stroke severity, risk factors, comorbidities and prehospital medications. TrOPN-N expression was significantly correlated with the expressions of the other biomarkers (OPN: ρ=0.41, P<0.01; S100B: ρ=0.35, P=0.01; and D-dimer: ρ=0.45, P<0.01). TrOPN-N level was higher in the diabetic (P=0.02) than in the non-diabetic patients, and in the statin users (P=0.02) than in the non-statin users. The S100B and D-dimer levels positively correlated with the National Institutes of Health Stroke Scale scores on admission (ρ=0.31, P=0.04 and ρ=0.38, P=0.02, respectively), and D-dimer level correlated with stroke size (ρ=0.44, P=0.03).
Biomarker profiles based on different stroke etiologies
The biomarkers showed significant differences between the atherothrombotic and non-atherothrombotic groups as follows: trOPN-N (median (interquartile range): 5.83 (0.0–8.6 ) vs. 0.0 (0.0–3.3) pmol l−1, P=0.03) and MMP-9 (544 (322–749 ) vs. 343 (254–485) ng ml−1, P=0.01; Table 3). Negative trOPN-N values were found in 16 cases (50%) of the non-atherothrombotic subtype and 8 cases (29%) of the atherothrombotic subtype. OPN, D-dimer and S100B levels did not differ significantly between the two clinical subtypes.
Biomarker cutoff values and odds ratios
The cutoff values obtained with the Youden Index from the receiver-operating characteristic curves were as follows: trOPN-N, >5.47 pmol l−1 (sensitivity, specificity and c-statistic: 0.54, 0.91 and 0.72, respectively) and MMP-9, >605 ng ml−1 (0.78, 0.67 and 0.71, respectively). By performing a logistic regression analysis adjusted for the prevalences of hypertension, diabetes and dyslipidemia, the independent association between two biomarkers and the atherothrombotic group was clarified, with the following values: trOPN-N, >5.47 pmol l−1 (odds ratio (OR), 11.7; 95% confidence interval (CI), 2.10–64.87; P=0.005) and MMP-9, >605 ng ml−1 (OR, 9.92; 95% CI, 1.89–52.24; P=0.007).
Next, we separately analyzed the different times from symptom onset to hospitalization as follows: within 3 h, within 12 h and within 24 h (all patients). Within 3 h (n=27), trOPN-N expression remained associated with the atherothrombotic group (OR, 15.9; 95% CI, 1.13–221; P=0.04), but MMP-9 expression did not (OR, 7.03; 95% CI, 0.46–106; P=0.16; Figure 2). MMP-9 level measured within 12 h newly showed a significant association with the atherothrombotic group (OR, 10.0; 95% CI, 1.33–75.3; P=0.03).
Logistic regression analysis results showing the association between blood biomarkers and the diagnosis of atherothrombotic ischemic stroke. Data are divided according to the timing of biomarker measurement from stroke onset. The upper three lines show thrombin-cleaved osteopontin N-terminal (trOPN-N), and the lower three, matrix metalloproteinase-9 (MMP-9). Values are expressed as medians, and horizontal lines represent the 95% confidence intervals, within 24 h (all patients), within 12 h and within 3 h.
Discussion
The main results of our study are as follows: (1) trOPN-N and MMP-9 levels were higher in the atherothrombotic than in the non-atherothrombotic ischemic stroke subtype and (2) only trOPN-N was significantly elevated in the acute phase (i.e., within 3 h after symptom onset) in the atherothrombotic subtype.
Different implications of OPN and trOPN-N expressions for atherosclerosis
OPN is an extracellular matrix protein that is localized around calcified or inflammatory tissue. It is secreted by many cell types such as lymphocytes, macrophages, endothelial cells and vascular smooth muscle cells. It exacerbates inflammation through the recruitment of macrophages and the regulation of cytokine production in macrophages, dendritic cells and T-cells.20 OPN has several protease cleavage sites and cell-interacting domains. OPN has two integrin-binding motifs, RGD and SVVYGLR. Only after thrombin cleavage is SVVYGLR revealed in trOPN-N, and SVVYGLR works as a ligand for integrin α9β1, α4β1 and α1β7.20 These integrins are expressed in activated macrophages in rheumatoid arthritis and introduce further inflammatory cytokines and chemokines.21 We previously reported that trOPN-N expression was observed only in vulnerable plaques in the atherosclerotic carotid artery.13 Wolak et al.22 also reported that inflammation severity correlated only with trOPN-N expression, not with full-length or C-terminal OPN in carotid specimens. trOPN-N expression is observed not only inside of the plaque but also in blood when the plaque ruptures. TrOPN-N level has been shown to be elevated significantly after carotid artery stenting, especially in patients with symptomatic ischemic stroke.13
In this study, trOPN-N levels were higher in the atherothrombotic subtype than in the non-atherothrombotic subtype, but OPN levels did not significantly differ between the two groups. Considering that the main pathophysiological mechanism of atherothrombotic stroke is artery-to-artery infarction due to plaque rupture,23 we can hypothesize that blood trOPN-N originated from vulnerable plaques. TrOPN-N levels were higher in the patients with than in those without diabetes mellitus (Table 2). These results indicate that the atherothrombotic group had more patients with diabetes mellitus and dyslipidemia than the non-atherothrombotic group. Even after adjusting for these risk factors, trOPN-N expression remained an independent determinant of the atherothrombotic subtype (Figure 2). Notably, the trOPN-N level in the non-atherothrombotic group (0.00 (0.00–3.30) pmol l−1) was the same as that in patients with essential hypertension and no history of cardiovascular disease or medical treatment (2.09 (0.00–3.81) pmol l−1).13 Furthermore, according to its high specificity (0.91) for the atherothrombotic group, high trOPN-N levels would explain pathological situations.
Diagnostic biomarker that explains atherosclerotic and thrombotic states
In this study, trOPN-N level showed a positive correlation with OPN and D-dimer levels. This result is compatible because OPN expression reflects plaque burden12 and D-dimer expression is a marker of coagulation activation in circulating blood.24 This result satisfies the requirement that a marker of atherothrombotic ischemic stroke should reflect both atherosclerosis and thrombosis. The atherothrombotic subtype is caused by a cerebral embolism in all vessels, from the aortic arch to the major cerebral arteries.25, 26, 27 Systemic trOPN levels reflect an atherothrombotic situation.
TrOPN-N expression in acute atherothrombotic stroke
TrOPN-N level was increased in the atherothrombotic subtype within 3 h after symptom onset. It is interesting that large differences in ORs and 95% CIs were found between the groups (<3 , <12 h and all <24 h; Figure 2) and that no relationship was found with symptom duration (Table 2). Our previous report also supports these data because after carotid artery stenting, trOPN-N expression could be detected within 3 h.13 Thrombolysis is unquestionably the first choice of treatment of acute ischemic stroke, regardless of whether it is of the atherothrombotic or cardiogenic subtype. Furthermore, Yoon et al.28 reported that emergent intracranial angioplasty is feasible and yields a high rate of revascularization and favorable outcomes in patients with the atherothrombotic subtype compared with those with other subtypes. Diagnosis of the stroke subtype is important for selecting the appropriate treatment. As trOPN-N expression was associated with stroke subtype in the early phase, it appears useful for treatment selection.
Pathophysiological mechanism of ischemic stroke in relation to MMP-9 expression
MMP-9 level was higher in the atherothrombotic group. One reason is that MMP-9 expression is also a marker of atherosclerosis.29 Indeed, the atherothrombotic group showed a higher prevalence of stroke risk factors overall (Table 1), and MMP-9 levels might reflect this. However, MMP-9 expression was only recognized as a significant biomarker after including data from blood samples obtained within 12 h of symptom onset (Figure 2). In a rat ischemic stroke model30 and in patients with ischemic stroke, MMP-9 levels began to increase after 12 h.31 These reports support our result that MMP-9 level could not distinguish atherothrombosis subtype in the super-acute phase. Thus, MMP-9 level is not suitable as a diagnostic marker of the atherothrombotic subtype in acute settings.
S100B and D-dimer: biomarkers of ischemic stroke
Several reports indicated that S100B and D-dimer levels correlated with stroke volume and/or functional severity.32, 33 In our study, they also correlated with stroke volume and/or National Institutes of Health Stroke Scale score. However, as both showed no difference between the atherothrombotic and non-atherothrombotic subtypes, they were not found to be suitable diagnostic biomarkers in this study.
Study limitations
This study had several methodological difficulties. First, our population was relatively small, and we omitted the undetermined etiology and ‘other determined etiologies’ stroke subtypes from the final analysis. In the future, studies with a large number of participants that enables the inclusion of these heterogeneous populations are needed. Second, even though the trOPN-N level significantly differed between the atherothrombotic and non-atherothrombotic subtypes, the trOPN-N levels of one-third of the patients were undetectable because of the low sensitivity of microplate-based enzyme-linked immunosorbent assay. However, we previously reported the usefulness of a capillary-based enzyme-linked immunosorbent assay system for trOPN-N, which can measure as little as 4 μl of plasma.34 It would be a useful tool for measuring trOPN-N levels in a large clinical trial. Finally, we did not measure brain natriuretic peptide levels for all patients, so the sensitivity/specificity directory of trOPN-N was difficult to compare with that of a well-known diagnostic biomarker of ischemic stroke subtype. It may be useful to measure both brain natriuretic peptide and trOPN-N levels for distinguishing between the cardiogenic and atherothrombotic subtypes.
Conclusion
The results of this study suggest that trOPN-N level has the potential to distinguish between the ischemic stroke atherothrombotic subtypes, especially in the acute phase.
References
Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ishimitsu T, Ito M, Ito S, Itoh H, Iwao H, Kai H, Kario K, Kashihara N, Kawano Y, Kim-Mitsuyama S, Kimura G, Kohara K, Komuro I, Kumagai H, Matsuura H, Miura K, Morishita R, Naruse M, Node K, Ohya Y, Rakugi H, Saito I, Saitoh S, Shimada K, Shimosawa T, Suzuki H, Tamura K, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Umemura S, Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2014). Hypertens Res 2014; 37: 253–390.
Toyoda K . Epidemiology and registry studies of stroke in Japan. J Stroke 2013; 15: 21–26.
Tanizaki Y, Kiyohara Y, Kato I, Iwamoto H, Nakayama K, Shinohara N, Arima H, Tanaka K, Ibayashi S, Fujishima M . Incidence and risk factors for subtypes of cerebral infarction in a general population: The Hisayama Study. Stroke 2000; 31: 2616–2622.
Kimura K, Kazui S, Minematsu K, Yamaguchi T, Japan Multicenter StrokeInvestigator’s Collaboration. Analysis of 16922 patients with acute ischemic stroke and transient ischemic attack in japan: a hospital-based prospective registration study. Cerebrovasc Dis 2004; 18: 47–56.
Saji N, Kimura K, Yagita Y, Kawarai T, Shimizu H, Kita Y . Comparison of arteriosclerotic indicators in patients with ischemic stroke: ankle-brachial index, brachial-ankle pulse wave velocity and cardio-ankle vascular index. Hypertens Res 2015; 38: 323–328.
Moroney JT, Bagiella E, Paik MC, Sacco RL, Desmond DW . Risk factors for early recurrence after ischemic stroke: the role of stroke syndrome and subtype. Stroke 1998; 29: 2118–2124.
Gupta R, Tayal AH, Levy EI, Cheng-Ching E, Rai A, Liebeskind DS, Yoo AJ, Hsu DP, Rymer MM, Zaidat OO, Lin R, Natarajan SK, Nogueira RG, Nanda A, Tian M, Hao Q, Abou-Chebl A, Kalia JS, Nguyen TN, Chen M, Jovin TG . Intra-arterial thrombolysis or stent placement during endovascular treatment for acute ischemic stroke leads to the highest recanalization rate: results of a multicenter retrospective study. Neurosurgery 2011; 68: 1618–1622; discussion 1622–1613.
Hussain MS, Lin R, Cheng-Ching E, Jovin TG, Moskowitz SI, Bain M, Horowitz M, Gupta R . Endovascular treatment of carotid embolic occlusions has a higher recanalization rate compared with cardioembolic occlusions. J Neurointerv Surg 2010; 2: 71–73.
Laskowitz DT, Kasner SE, Saver J, Remmel KS, Jauch EC, BRAIN Study Group. Clinical usefulness of a biomarker-based diagnostic test for acute stroke: the Biomarker Rapid Assessment in Ischemic Injury (BRAIN) study. Stroke 2009; 40: 77–85.
Mazzali M, Kipari T, Ophascharoensuk V, Wesson JA, Johnson R, Hughes J . Osteopontin: a molecule for all seasons. QJM 2002; 95: 3–13.
Kurata M, Okura T, Watanabe S, Fukuoka T, Higaki J . Osteopontin and carotid atherosclerosis in patients with essential hypertension. Clin Sci (Lond) 2006; 111: 319–324.
Golledge J, Muller J, Shephard N, Clancy P, Smallwood L, Moran C, Dear AE, Palmer LJ, Norman PE . Association between osteopontin and human abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol 2007; 27: 655–660.
Kurata M, Okura T, Kumon Y, Tagawa M, Watanabe H, Nakahara T, Tatsuhiko M, Higaki J, Nose M . Plasma thrombin-cleaved osteopontin elevation after carotid artery stenting in symptomatic ischemic stroke patients. Hypertens Res 2012; 35: 207–212.
Spagnoli LG, Mauriello A, Sangiorgi G, Fratoni S, Bonanno E, Schwartz RS, Piepgras DG, Pistolese R, Ippoliti A, Holmes DR Jr. . Extracranial thrombotically active carotid plaque as a risk factor for ischemic stroke. JAMA 2004; 292: 1845–1852.
Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE III . Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24: 35–41.
Lee LJ, Kidwell CS, Alger J, Starkman S, Saver JL . Impact on stroke subtype diagnosis of early diffusion-weighted magnetic resonance imaging and magnetic resonance angiography. Stroke 2000; 31: 1081–1089.
Goldstein LB, Samsa GP . Reliability of the National Institutes of Health Stroke Scale. Extension to non-neurologists in the context of a clinical trial. Stroke 1997; 28: 307–310.
van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J . Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988; 19: 604–607.
Fluss R, Faraggi D, Reiser B . Estimation of the Youden Index and its associated cutoff point. Biom J 2005; 47: 458–472.
Scatena M, Liaw L, Giachelli CM . Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol 2007; 27: 2302–2309.
Yamamoto N, Sakai F, Kon S, Morimoto J, Kimura C, Yamazaki H, Okazaki I, Seki N, Fujii T, Uede T . Essential role of the cryptic epitope SLAYGLR within osteopontin in a murine model of rheumatoid arthritis. J Clin Invest 2003; 112: 181–188.
Wolak T, Sion-Vardi N, Novack V, Greenberg G, Szendro G, Tarnovscki T, Nov O, Shelef I, Paran E, Rudich A . N-terminal rather than full-length osteopontin or its C-terminal fragment is associated with carotid-plaque inflammation in hypertensive patients. Am J Hypertens 2013; 26: 326–333.
Rothwell PM, Gibson R, Warlow CP . Interrelation between plaque surface morphology and degree of stenosis on carotid angiograms and the risk of ischemic stroke in patients with symptomatic carotid stenosis. On behalf of the European Carotid Surgery Trialists’ Collaborative Group. Stroke 2000; 31: 615–621.
Lowe GD, Rumley A . Use of fibrinogen and fibrin D-dimer in prediction of arterial thrombotic events. Thromb Haemost 1999; 82: 667–672.
Tunick PA, Kronzon I . Atheromas of the thoracic aorta: clinical and therapeutic update. J Am Coll Cardiol 2000; 35: 545–554.
Wasserman BA . Advanced contrast-enhanced MRI for looking beyond the lumen to predict stroke: building a risk profile for carotid plaque. Stroke 2010; 41 (10 Suppl): S12–S16.
Qureshi AI, Feldmann E, Gomez CR, Johnston SC, Kasner SE, Quick DC, Rasmussen PA, Suri MF, Taylor RA, Zaidat OO . Intracranial atherosclerotic disease: an update. Ann Neurol 2009; 66: 730–738.
Yoon W, Kim SK, Park MS, Kim BC, Kang HK . Endovascular treatment and the outcomes of atherosclerotic intracranial stenosis in patients with hyperacute stroke. Neurosurgery 2015; 76: 680–686; discussion 686.
Hansson J, Vasan RS, Arnlov J, Ingelsson E, Lind L, Larsson A, Michaelsson K, Sundstrom J . Biomarkers of extracellular matrix metabolism (MMP-9 and TIMP-1) and risk of stroke, myocardial infarction, and cause-specific mortality: cohort study. PLoS ONE 2011; 6: e16185.
Rosenberg GA, Navratil M, Barone F, Feuerstein G . Proteolytic cascade enzymes increase in focal cerebral ischemia in rat. J Cereb Blood Flow Metab 1996; 16: 360–366.
Montaner J, Alvarez-Sabin J, Molina C, Angles A, Abilleira S, Arenillas J, Gonzalez M, Monasterio J . Matrix metalloproteinase expression after human cardioembolic stroke: temporal profile and relation to neurological impairment. Stroke 2001; 32: 1759–1766.
Dassan P, Keir G, Brown MM . Criteria for a clinically informative serum biomarker in acute ischaemic stroke: a review of S100B. Cerebrovasc Dis 2009; 27: 295–302.
Zi WJ, Shuai J . Plasma D-dimer levels are associated with stroke subtypes and infarction volume in patients with acute ischemic stroke. PLoS ONE 2014; 9: e86465.
Funano S, Henares TG, Kurata M, Sueyoshi K, Endo T, Hisamoto H . Capillary-based enzyme-linked immunosorbent assay for highly sensitive detection of thrombin-cleaved osteopontin in plasma. Anal Biochem 2013; 440: 137–141.
Acknowledgements
We thank Drs Katsusuke Kusunoki, Toshitaka Shiraishi and Kou Nakagawa for their contribution to the patient clinical information used in the study. This work was supported by a Kakenhi grant, Grant-in-Aid for Young Scientists (B) (24791505) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan Society for the Promotion of Science (JSPS) and the Japan Society for the Promotion of Science; and a Japan Heart Foundation Research Grant (to MK).
Author contributions
All the authors were involved in drafting the manuscript. MK had full access to all the data in the study and takes responsibility for the data and accuracy of the data analysis. Study conception and design: Saya O, YK and MK. Acquisition of data: SM, MT, HW and Shiro O. Analysis and interpretation of data: JH and TO.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ozaki, S., Kurata, M., Kumon, Y. et al. Plasma thrombin-cleaved osteopontin as a potential biomarker of acute atherothrombotic ischemic stroke. Hypertens Res 40, 61–66 (2017). https://doi.org/10.1038/hr.2016.110
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2016.110
Keywords
This article is cited by
-
Angiotensin 1–7, but not the thrombin-cleaved osteopontin C-terminal fragment, attenuates osteopontin-mediated macrophage-induced endothelial-cell inflammation
Inflammation Research (2018)
-
Plasma thrombin-cleaved osteopontin as a potential biomarker of acute atherothrombotic ischemic stroke: comments on data sparsity
Hypertension Research (2017)