Abstract
The (pro)renin receptor ((P)RR) is expressed in several tissues including kidney, heart and brain, and is thought to regulate the tissue renin–angiotensin system (RAS) through the non-proteolytic activation of prorenin. (P)RR is cleaved by furin to generate soluble (P)RR (s(P)RR), which is secreted into the extracellular space. s(P)RR is a candidate biomarker reflecting the status of the tissue RAS. Here, we investigated the relationship between background factors and serum s(P)RR levels. We measured s(P)RR levels in 122 patients with essential hypertension (EH) and assessed the relationships between background factors and s(P)RR levels. Serum s(P)RR levels were 19.0±4.9 ng ml–1. Single regression analyses showed that age (r=0.251, P<0.01), serum creatinine levels (r=0.229, P<0.05) and urinary angiotensinogen excretion (r=0.196, P<0.05) were positively correlated with s(P)RR levels, whereas estimated glomerular filtration rate (eGFR; r=−0.337, P<0.001) were negatively correlated. Multiple regression analyses of age, blood pressure (BP), hemoglobin A1c (HbA1c) and s(P)RR levels revealed that age and s(P)RR levels were negatively correlated with the eGFR (P<0.05). In patients with EH, serum s(P)RR levels correlated positively with renal function independent of age, BP and HbA1c. These findings support s(P)RR as a useful biomarker that reflects the status of the tissue RAS.
Similar content being viewed by others
Introduction
The (pro)renin receptor ((P)RR), which consists of 350 amino acids with a single transmembrane domain and binds preferentially to renin and prorenin, was identified in 2002.1 The (P)RR is widely expressed in various tissues, including the brain, heart and kidney. Once prorenin binds to (P)RR, it exerts two functions.1, 2 First, prorenin undergoes a conformational change without proteolytic cleavage to develop renin activity, which catalyzes the conversion of angiotensinogen (AGT) to angiotensin I. This process has a key role in regulating the tissue renin–angiotensin system (RAS). Second, (P)RR induces its own intracellular signaling transduction that activates the mitogen-activated protein kinase extracellular signal-regulated kinase 1/2 pathways independent of the RAS.3 (P)RR also has a physiological role in the assembly and function of vacuolar H+-ATPase (V-ATPase), an ATP-dependent proton pump that transports protons across plasma membranes and acidifies intracellular compartments.4
(P)RR is cleaved by furin to generate soluble (P)RR (s(P)RR) and M8–9, a residual hydrophobic truncated protein. The s(P)RR is secreted into the extracellular space and is ultimately found in blood and urine.5, 6 The s(P)RR also binds to prorenin and mediates the activation of prorenin in cell culture medium7, 8 and urine,6 suggesting that s(P)RR itself could have functional roles. Taken together, these findings highlight s(P)RR as a candidate biomarker for reflecting the tissue RAS status.
Recently, we developed an s(P)RR enzyme-linked immunosorbent assay kit to measure the concentration of s(P)RR in blood.9 We previously reported the usefulness of s(P)RR measurements in the clinical settings by showing that high circulating levels of s(P)RR at early pregnancy predict a subsequent elevation in blood pressure (BP) and that high concentrations at delivery are significantly associated with preeclampsia.10 Population-based clinical studies suggested that (P)RR gene polymorphisms may reflect BP levels.11, 12 Therefore, it is possible that activation of the tissue RAS by (P)RR is associated with the regulation of BP. However, the pathophysiology and clinical significance of blood s(P)RR levels in essential hypertension (EH) remains unclear. This study thus aimed to determine serum s(P)RR levels in patients with EH and to assess the relationship between background factors associated with hypertension including renal function and serum s(P)RR levels.
Methods
Subjects
Patients with EH who visited our outpatient clinic from October 2011 to January 2013 and age-matched normotensive subjects were included in this study. Hypertensive patients were either untreated or treated only with calcium channel blockers except cilnidipine, which inhibits L-type and N-type calcium channels and could affect the RAS.13, 14, 15 Patients who had suffered from a hemorrhagic stroke or cardiac infarction in the previous 6 months, pregnant women and those with apparent peripheral vascular, malignant disease or uncontrolled diabetes mellitus (hemoglobin A1c (HbA1c) of higher than 10.0%) were excluded. All participants were enrolled after obtaining informed consents approved by the ethical committee of Tokyo Women’s Medical University.
Background factors
At enrollment, information was collected on gender, age, body mass index and waist circumference. Waist circumference was measured at the height of the umbilicus while the patient was standing and after exhaling.
Office BP and pulse rate
Office BP and pulse rate (PR) were measured at an outpatient clinic with the patient in a sitting position after resting for at least 5 min. The first readings at each visit were used for this study.
Ambulatory BP monitoring
Ambulatory BP monitoring (ABPM) was performed with an automatic device, TM-2431 (A&D, Tokyo, Japan), which recorded BP (by the oscillometric method) and PR every 30 min from 0600 to 2200 hours and every 60 min during the rest of the day. Nighttime BP and PR were defined as the average of measurements recorded from the time when the patient went to bed until the time he/she got out of bed. Daytime BP and PR were defined as the average of measurements recorded during the rest of the day. The morning surge in systolic BP was calculated by subtracting the 2-h average systolic BP before waking from the 2-h average systolic BP after waking. The fall in nocturnal systolic BP (%) was calculated as 100 × (1–nighttime systolic BP/daytime systolic BP ratio).
Urinary examinations
Spot urine samples were obtained and concentrations of creatinine, albumin, Na+, metanephrine and normetanephrine were quantified by standardized assessment methods at our clinical laboratory center, and AGT levels were measured using a sandwich enzyme-linked immunosorbent assay as described.16 Excretion of albumin, Na+, metanephrine and normetanephrine, and AGT were evaluated by dividing these values by the creatinine concentration.
Blood examinations
Blood samples were taken while patients were sitting after at least 15 min of rest. Blood sugar, HbA1c, low-density lipoprotein cholesterol, high-density lipoprotein (HDL)-cholesterol, triglyceride, creatinine, uric acid and high-sensitivity C-reactive protein were measured by standard laboratory methods at our clinical laboratory center. Serum levels of s(P)RR (Takara Bio, Otsu City, Japan)9 and prorenin (Innovative Research, Novi, MI, USA) were measured using an enzyme-linked immunosorbent assay kit consisting of a solid-phase sandwich enzyme-linked immunosorbent assay with antibodies highly specific for each protein. Plasma renin activity (PRA) and plasma aldosterone concentration (PAC) were measured by a radioimmunoassay at an external laboratory (SRL, Tokyo, Japan). The estimated glomerular filtration rate (eGFR) was calculated using the following equation:
eGFR (ml min–1 per 1.73 m2)=194 × creatinine−1.094 × age−0.287 ( × 0.739 if female).17
Flow-mediated vasodilation
Percent changes in brachial artery diameter were calculated in response to increased flow-mediated vasodilation (FMD), an index of endothelial function, as previously described18, 19 by using UNEX EF38G (UNEX, Nagoya, Japan).
Cardio-ankle vascular and ankle-brachial indices
The cardio-ankle vascular index (CAVI), an index of arteriosclerosis, and the ankle-brachial index were measured using a VaSera VS-1500AN vascular screening system (Fukuda Denshi, Tokyo, Japan), as described previously.20 Patients with an ankle-brachial index <0.90 were excluded from the CAVI calculations.
Augmentation index and central systolic BP
The augmentation index (AI), an index of arteriosclerosis, and central systolic BP were measured using an automated tonometric device (HEM-9000AI; Omron Healthcare, Kyoto, Japan), as described previously.21, 22
Carotid intima-media thickness
Carotid intima-media thickness (IMT) was measured from carotid ultrasound examinations of the common carotid artery, bulb and internal carotid artery performed bilaterally using the Nemio XG ultrasound system (Toshiba, Tokyo, Japan). The examinations were conducted while the subjects were in a supine position with the head turned 45° from the site being scanned. Both carotid arteries were scanned longitudinally in order to visualize the IMT in the far wall of the artery, and the maximum IMT, an index of atherosis, was assessed.
Study protocol
Relationship between background factors, office BP and PR, central systolic BP, BP and PR data obtained by ABPM, urinary and blood data, FMD, CAVI, AI and maximum IMT and s(P)RR levels were examined by single or multiple regression analyses. Relationships between age and serum prorenin level, PRA and PAC, and those between eGFR and serum prorenin levels, PRA and PAC were assessed by single regression analyses.
Statistical analysis
All data are expressed as mean±s.d. Single regression analyses were performed to determine the correlation between background factors, office BP and PR, BP and PR data obtained by ABPM, urinary and blood data, physiological function tests and s(P)RR, and correlation between age and serum prorenin level, PRA and PAC, and that between eGFR and serum prorenin level, PRA and PAC. Power analyses were done by using G*Power 3.1.7.23 The r value was reported to be −0.275 for the relationship between serum s(P)RR level and eGFR in patients with chronic kidney disease in a previous study.24 A priori power analysis given this r value suggested that at least 98 patients are required to determine the correlation with a power of 80% between serum s(P)RR and eGFR in our patients. Multiple regression analyses were used to identify possible determinants. Non-paired Student’s t-tests and χ2-test were applied to compare two groups. The level of significance was defined as P<0.05.
Results
Characteristics of the study subjects
A total of 122 patients with EH were enrolled in this study. Table 1 details the background factors, comprising office BP and PR, central systolic BP, BP and PR data obtained by ABPM, urinary and blood data, and data from the physiological function tests. No patients showed an ankle-brachial index of <0.9. Taken together, these data indicated that most study subjects had EH with mild to moderate, but not severe, organ damage.
Age-matched 32 normotensive subjects (12 men) were enrolled as controls. The average age was 53±12 years, systolic BP was 117±9 mm Hg, diastolic BP was 72±8 mm Hg, blood sugar was 98.4±19.0 mg dl–1, HbA1c was 5.7±0.6%, low-density lipoprotein cholesterol was 106±32 mg dl–1, HDL-cholesterol was 72±21 mg dl–1, triglyceride was 123±98 mg dl–1 and eGFR was 85.0±21.3 ml min–1 per 1.73 m2. Both systolic BP and diastolic BP were significantly lower than those of essential hypertensive patients (P<0.0001 for both), and none of the other values were significantly different between patients with EH and normotensive subjects.
Serum s(P)RR levels
In patients with EH, the average serum s(P)RR levels were 19.0±4.9 ng ml–1 and the values were not different between male (18.8±4.6 ng ml–1, n=56) and female (19.2±5.2, n=66) patients, or between patients without medication (19.0±4.6, n=52) and those with treated with calcium channel blocker (17.8±4.3, n=70). In normotensive subjects, the average serum s(P)RR levels were 20.3±4.0 ng ml–1, and these values were not significantly different compared with those of essential hypertensive patients. There were no significant difference between men (20.1±2.1 ng ml–1, n=12) and women (20.4±4.2 ng ml–1, n=20) in the values of serum (P)RR.
Relationships between background factors and RAS components
In patients with EH, age was significantly positively correlated with the serum level of s(P)RR (Table 2 and Figure 1a), but was not significantly correlated with serum prorenin levels (r=0.113, P=0.291). Age was significantly negatively correlated with PRA (r=−0.298, P<0.005) and PAC (r=−0.332, P<0.0005). Figure 1b shows data comparing s(P)RR levels between male and female subjects for each age group. Serum s(P)RR levels were significantly higher in men aged in their 70s than those in their 50s and significantly higher in women in their 60s than those in their 30s. Serum s(P)RR levels were not significantly different between men and women in any age group. As there was no gender difference in s(P)RR levels further analyses were performed without dividing patients into male and female groups. No data related to BP or PR showed a relationship with s(P)RR levels (Table 2).
Relationship between age and serum soluble (pro)renin receptor (s(P)RR) level. (a) Scattergram showing relationship between age and serum s(P)RR level. (b) Comparison of serum s(P)RR levels between male and female subjects for each age group. *P<0.05 compared with 30s, **P<0.05 compared with 50s. NS, not significant; y.o, years old.
Correlation between urinary or blood data and serum s(P)RR levels
In patients with EH, urinary AGT excretion was weakly but significantly positively correlated with the serum level of s(P)RR (Table 2 and Figure 2a). When patients were divided into low s(P)RR (<17 ng ml–1) and high s(P)RR (⩾17 mg ml–1) groups, urinary AGT excretion was significantly higher in s(P)RR group than in low s(P)RR group (Figure 2b). Among the blood data, HDL-cholesterol and triglyceride levels showed weak but significantly negative and significantly positive relationships, respectively, with serum s(P)RR levels (Table 2). There were no significant relationships between s(P)RR levels and serum prorenin levels, PRA and PAC (Table 2).
Relationship between serum soluble (pro)renin receptor (s(P)RR) level and urinary angiotensinogen (AGT) excretion. (a) Scattergrams showing relationship between serum s(P)RR level and urinary AGT excretion. (b) Comparison of urinary AGT excretion between low and high s(P)RR groups. *P<0.05 compared with low s(P)RR group.
Relationships between renal function and RAS components
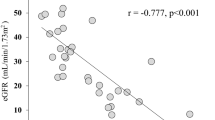
In patients with EH, serum creatinine levels (Table 2) and eGFR (Table 2 and Figure 3) showed significantly positive and significantly negative relationships, respectively, with serum s(P)RR levels. In contrast, there were no significant relationships between eGFR and serum prorenin levels (r=−0.051, P=0.611), PRA (r=−0.072, P=0.902) or PAC (r=0.133, P=0.432).
It is well known that age, BP and glucose metabolism affect renal function. To determine if the relationship between eGFR and serum s(P)RR levels is independent of these factors, multiple regression analysis testing age, systolic BP, HbA1c and serum s(P)RR levels as independent variables was performed. Serum s(P)RR levels in addition to age were negatively correlated with eGFR (Table 3).
There was a significant positive relationship between eGFR and serum s(P)RR levels in normotensive subjects as well (r=−0.576, P<0.001; Figure 3). Stepwise multiple regression analysis in all subjects including normotensives and hypertensives revealed that eGFR (P<0.0001) but not existence of hypertension was significantly correlated with serum s(P)RR levels, indicating that the relationship between eGFR and serum s(P)RR levels is independent of existence of hypertension.
Relationship between physiological function tests and serum s(P)RR levels
In patients with EH, there were no significant relationships between serum s(P)RR levels and FMD, AI or the carotid maximum IMT (Table 2). On the other hand, there was a significant positive relationship between CAVI and serum s(P)RR levels (Table 2), suggesting an interaction between arteriosclerosis and serum s(P)RR levels. Multiple regression analyses were performed to determine if the relationship between CAVI and serum s(P)RR levels was independent of age, BP or glucose metabolism. In model 1, where we tested age, systolic BP, HbA1c and serum s(P)RR levels as independent variables, only age was related to CAVI (Table 4). Similarly, in model 2, where the independent variables were limited to age and serum s(P)RR levels, age but not serum s(P)RR level was related to CAVI (Table 4).
Discussion
This study demonstrated four major findings regarding serum s(P)RR levels in patients with EH. First, serum s(P)RR levels positively correlated with age, with no obvious gender differences. Second, serum s(P)RR levels were associated with lipid metabolism dysfunction. Third, serum s(P)RR levels correlated with renal function independent of age, BP and glucose metabolism. Finally, serum s(P)RR levels were positively related to CAVI values and this relationship was considered age dependent.
Relationship between background factors and RAS components
Among background factors, age was significantly positively correlated with s(P)RR levels and significantly negatively correlated with PRA and PAC, in accordance with previous reports.25, 26, 27 Renin downregulates (P)RR expression by a process involving the transcription factor promyelocytic zinc-finger protein.28 One may speculate that serum s(P)RR levels are affected by PRA. However, there was no significant relationship between serum s(P)RR levels and PRA in this study. A tissue-specific conditional knockout approach in recent studies elucidated the functional relevance of (P)RR as a proton pump protein. The cardiomyocyte-specific (P)RR knockout mice4 and the podocyte-specific (P)RR knockout mice29 die early due to fulminant heart failure and kidney failure, respectively, as a result of V-ATPase dysfunction causing impaired autophagy, which is observed in the process of aging. It can be speculated therefore that serum s(P)RR levels are elevated because of increases in (P)RR expression resulting from V-ATPase activation to compensate impaired autophagy in the aging process. Further studies are required to test this presumption. Our study also showed that s(P)RR levels were not significantly different between men and women at any age group, excluding the possibility that a gender difference exists in the s(P)RR levels. Serum (P)RR levels were not significantly different between patients with EH and normotensive subjects. Serum (P)RR levels were not related with any BP data. Collectively, we found no evidence of a relationship between s(P)RR levels and BP levels in the present cross-sectional study. To further examine the relationship between BP and s(P)RR levels, we are currently investigating changes in s(P)RR levels induced by different types of antihypertensive agents.
Relationship between lipid profiles and serum s(P)RR levels
Intriguingly, HDL-cholesterol and triglyceride levels showed weak, but significantly negative and significantly positive relationships, respectively, with serum s(P)RR levels, suggesting the presence of interactions between lipid metabolism dysfunction and serum s(P)RR levels. The reason for these associations remains to be elucidated. However, it is established that adipose tissue harbors all components of the RAS including (P)RR.30, 31 Achard et al.32 recently reported that overfeeding and a high-fat diet increased visceral fat mass and adipose (P)RR expression in rats, suggesting that patients with dyslipidemia could have increased expression of (P)RR in adipose tissues that causes an elevation in serum (P)RR levels. Our data unfortunately failed to show a significant relationship between serum s(P)RR levels and waist circumference, which is an indirect marker for visceral fat mass (Table 2). We are currently examining the relationship between visceral fat mass and s(P)RR level in another series of patients to test this assumption. The relationships between HDL-cholesterol and triglycerides and serum s(P)RR levels may suggest a relationship between serum s(P)RR levels and vascular damages. Assessment of relationship between vascular factors such as adhesions and serum s(P)RR levels may help understanding this relationship in future studies.
Relationship between renal function and RAS components
Serum s(P)RR level was associated with decreased eGFR independent of age, BP and glucose metabolism. Although the reasons for this phenomenon remain unclear, there are several possible explanations. First, elevated serum s(P)RR levels may reflect decreased clearance of s(P)RR from the kidney, although whether this 28-kDa protein is filtered from the glomeruli or whether it is excreted into the urine without being absorbed from the tubules is unknown. Investigations of the relationships between eGFR, serum (P)RR concentration and urinary (P)RR concentration could help clarify these points. Second, elevated serum s(P)RR levels may reflect an increase in expression of (P)RR in the kidneys, which could in turn increase the local production of angiotensin II by activating prorenin. Increased expression of (P)RR was observed in the remnant kidneys of 5/6 nephrectomized rats.33 We have reported that a (P)RR blocker handle region peptide inhibited the development and progression of renal damage in hypertensive rats.34 Urinary protein excretion was reported to be significantly positively related with serum s(P)RR levels in chronic kidney disease patients.24 In this study, serum s(P)RR levels were significantly positively related with urinary AGT excretion (Figure 2), a biomarker for intrarenal RAS.35, 36 In addition, in our preliminary study with a subgroup of essential hypertensive patients, serum s(P)RR levels were significantly positively related with urinary excretion of 8-hydroxydeoxyguanosine (n=22, β=0.419, P<0.05), which is a marker of oxidative stress causing renal damages, independently with eGFR. All these experimental and clinical data support the presumption that increased expression of (P)RR in the kidney may lead to both elevated serum s(P)RR levels and renal dysfunction. In this study, no significant relationship between urinary albumin expression and serum s(P)RR levels were observed (Table 2). In the kidney, (P)RR is mainly expressed in tubular cells and collecting duct cells and the expression levels in glomeruli cells such as podocytes and endothelial cells are very low.37 Therefore, it is considered that this result does not exclude the presumption because increased (P)RR expression in the kidney may have stronger impacts on tubulointerstitial injuries than occurrence of albuminuria, which is initially caused by damages in glomeruli cells. Third, increased serum s(P)RR levels may be the result of enhanced cleavage of (P)RR by furin. It is possible that increased cleavage of (P)RR inhibits (P)RR-dependent V-ATPase activity and impairs autophagy in the kidney to progress kidney aging and ultimately a decrease in eGFR.
Relationship between CAVI and serum s(P)RR levels
CAVI, an index of arteriosclerosis, showed a significantly positive relationship with serum s(P)RR level. However, FMD, an index of endothelial dysfunction, and other indices of atherosclerosis, including AI and carotid maximum IMT, showed no significant relationships with serum s(P)RR level. Furthermore, the relationship between CAVI and serum s(P)RR levels was not detected after correcting for age, systolic BP and HbA1c (model 1 of Table 4) or age alone (model 2 of Table 4). Therefore, it is likely that the relationship between CAVI and serum s(P)RR levels observed in the single regression analysis was affected by the relationship between age and serum s(P)RR level.
Limitations
Several limitations to this study warrant mention. First, we could not determine the source of s(P)RR in serum. In addition, whether s(P)RR is simply a biomarker for the status of tissue RAS or whether it has a functional role in activating the tissue RAS remains unclear. Second, the number of patients tested was small. Post hoc power analyses revealed that the power (1-β) was high enough for age (0.802) and eGFR (0.970), but not for HDL-cholesterol (0.792), triglyceride (0.598) or CAVI (0.688). Large-scale studies are needed to clarify the mechanisms underlying the role of serum s(P)RR levels in hypertension. Third, our findings are basically based on the relationships between serum s(P)RR levels and background factors. Longitudinal studies may reveal the impacts of serum s(P)RR levels on the progression of organ damages and prognosis of the patients.
Conclusions
In conclusion, there is a significant relationship between serum s(P)RR levels in patients with EH and renal function that is independent of age, BP and glucose metabolism. An increase in the intrarenal RAS status is thought to underlie the progression of kidney damage. Serum s(P)RR could therefore serve as a useful biomarker reflecting the tissue RAS status in clinical settings. Furthermore, there may be associations between age or lipid metabolism dysfunction and serum s(P)RR levels. Future studies, including investigations to determine the source of serum s(P)RR in various pathological conditions, are needed for serum s(P)RR to be validated a useful biomarker for tissue RAS.
References
Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD . Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 2002; 109: 1417–1427.
Nguyen G, Burckle CA, Sraer JD . Renin/prorenin-receptor biochemistry and functional significance. Curr Hypertens Rep 2004; 6: 129–132.
Sakoda M, Ichihara A, Kaneshiro Y, Takemitsu T, Nakazato Y, Nabi AH, Nakagawa T, Suzuki F, Inagami T, Itoh H . (Pro)renin receptor-mediated activation of mitogen-activated protein kinases in human vascular smooth muscle cells. Hypertens Res 2007; 30: 1139–1146.
Kinouchi K, Ichihara A, Sano M, Sun-Wada GH, Wada Y, Kurauchi-Mito A, Bokuda K, Narita T, Oshima Y, Sakoda M, Tamai Y, Sato H, Fukuda K, Itoh H . The (pro)renin receptor/ATP6AP2 is essential for vacuolar H+-ATPase assembly in murine cardiomyocytes. Circ Res 2010; 107: 30–34.
Cousin C, Bracquart D, Contrepas A, Corvol P, Muller L, Nguyen G . Soluble form of the (pro)renin receptor generated by intracellular cleavage by furin is secreted in plasma. Hypertension 2009; 53: 1077–1082.
Gonzalez AA, Lara LS, Luffman C, Seth DM, Prieto MC . Soluble form of the (pro)renin receptor is augmented in the collecting duct and urine of chronic angiotensin II-dependent hypertensive rats. Hypertension 2011; 57: 859–864.
Biswas KB, Nabi AN, Arai Y, Nakagawa T, Ebihara A, Ichihara A, Watanabe T, Inagami T, Suzuki F . Aliskiren binds to renin and prorenin bound to (pro)renin receptor in vitro. Hypertens Res 2010; 33: 1053–1059.
Yoshikawa A, Aizaki Y, Kusano K, Kishi F, Susumu T, Iida S, Ishiura S, Nishimura S, Shichiri M, Senbonmatsu T . The (pro)renin receptor is cleaved by ADAM19 in the Golgi leading to its secretion into extracellular space. Hypertens Res 2011; 34: 599–605.
Maruyama N, Segawa T, Kinoshita N, Ichihara A . Novel sandwich ELISA for detecting the human soluble (pro)renin receptor. Front Biosci 2013; 5: 583–590.
Watanabe N, Bokuda K, Fujiwara T, Suzuki T, Mito A, Morimoto S, Jwa SC, Egawa M, Arai Y, Suzuki F, Sago H, Ichihara A . Soluble (pro)renin receptor and blood pressure during pregnancy: a prospective cohort study. Hypertension 2012; 60: 1250–1256.
Hirose T, Hashimoto M, Totsune K, Metoki H, Asayama K, Kikuya M, Sugimoto K, Katsuya T, Ohkubo T, Hashimoto J, Rakugi H, Takahashi K, Imai Y . Association of (pro)renin receptor gene polymorphism with blood pressure in Japanese men: the Ohasama study. Am J Hypertens 2009; 22: 294–299.
Ott C, Schneider MP, Delles C, Schlaich MP, Hilgers KF, Schmieder RE . Association of (pro)renin receptor gene polymorphism with blood pressure in Caucasian men. Pharmacogenetics Genomics 2011; 21: 347–349.
Kaneshiro Y, Ichihara A, Sakoda M, Kurauchi A, Takemitsu T, Itoh H . Cilnidipine and Telmisartan similarly improves vascular damage in hypertensive patients. Clin Med Cardiol 2007; 1: 1–11.
Ueno D, Masaki T, Gotoh K, Chiba S, Kakuma T, Yoshimatsu H . Cilnidipine regulates glucose metabolism and levels of high-molecular adiponectin in diet-induced obese mice. Hypertens Res 2013; 36: 196–201.
Soeki T, Kitani M, Kusunose K, Yagi S, Taketani Y, Koshiba K, Wakatsuki T, Orino S, Kawano K, Sata M . Renoprotective and antioxidant effects of cilnidipine in hypertensive patients. Hypertens Res 2012; 35: 1058–1062.
Katsurada A, Hagiwara Y, Miyashita K, Satou R, Miyata K, Ohashi N, Navar LG, Kobori H . Novel sandwich ELISA for human angiotensinogen. Am J Physiol Renal Physiol 2007; 293: F956–F960.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A . Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992.
Morimoto S, Yurugi T, Aota Y, Sakuma T, Jo F, Nishikawa M, Iwasaka T, Maki K . Prognostic significance of ankle-brachial index, brachial-ankle pulse wave velocity, flow-mediated dilation, and nitroglycerin-mediated dilation in end-stage renal disease. Am J Nephrol 2009; 30: 55–63.
Watanabe K, Mori T, Iwasaki A, Kimura C, Matsushita H, Shinohara K, Wakatsuki A . Increased oxygen free radical production during pregnancy may impair vascular reactivity in preeclamptic women. Hypertens Res 2013; 36: 356–360.
Saji N, Kimura K, Shimizu H, Kita Y . Silent brain infarct is independently associated with arterial stiffness indicated by cardio-ankle vascular index (CAVI). Hypertens Res 2012; 35: 756–760.
Fujime M, Tomimatsu T, Okaue Y, Koyama S, Kanagawa T, Taniguchi T, Kimura T . Central aortic blood pressure and augmentation index during normal pregnancy. Hypertens Res 2012; 35: 633–638.
Takase H, Dohi Y, Kimura G . Distribution of central blood pressure values estimated by Omron HEM-9000AI in the Japanese general population. Hypertens Res 2013; 36: 50–57.
Faul F, Erdfelder E, Lang AG, Buchner A . G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007; 39: 175–191.
Hamada K, Taniguchi Y, Shimamura Y, Inoue K, Ogata K, Ishihara M, Horino T, Fujimoto S, Ohguro T, Yoshimoto Y, Ikebe M, Yuasa K, Hoshino E, Iiyama T, Ichihara A, Terada Y . Serum level of soluble (pro)renin receptor is modulated in chronic kidney disease. Clin Exp Nephrol 2013; 17: 848–856.
Weidmann P, De Myttenaere-Bursztein S, Maxwell MH, de Lima J . Effect on aging on plasma renin and aldosterone in normal man. Kidney Int 1975; 8: 325–333.
Crane MG, Harris JJ . Effect of aging on renin activity and aldosterone excretion. J Lab Clin Med 1976; 87: 947–959.
Messerli FH, Sundgaard-Riise K, Ventura HO, Dunn FG, Glade LB, Frohlich ED . Essential hypertension in the elderly: haemodynamics, intravascular volume, plasma renin activity, and circulating catecholamine levels. Lancet 1983; 2: 983–986.
Shefe JHea . A novel signal transduction cascade involving direct physical interaction of the renin/prorenin receptor with the transcription factor promeylocyctic zinc finger protein. Circ Res 2006; 99: 1355–1366.
Oshima Y, Kinouchi K, Ichihara A, Sakoda M, Kurauchi-Mito A, Bokuda K, Narita T, Kurosawa H, Sun-Wada GH, Wada Y, Yamada T, Takemoto M, Saleem MA, Quaggin SE, Itoh H . Prorenin receptor is essential for normal podocyte structure and function. J Am Soc Nephrol 2011; 22: 2203–2212.
Engeli S, Negrel R, Sharma AM . Physiology and pathophysiology of the adipose tissue renin-angiotensin system. Hypertension 2000; 35: 1270–1277.
Achard V, Boullu-Ciocca S, Desbriere R, Nguyen G, Grino M . Renin receptor expression in human adipose tissue. Am J Physiol Regul Integr Comp Physiol 2007; 292: R274–R282.
Achard V, Tassistro V, Boullu-Ciocca S, Grino M . Expression and nutritional regulation of the (pro)renin receptor in rat visceral adipose tissue. J Endocrinol Invest 2011; 34: 840–846.
Hirose T, Mori N, Totsune K, Morimoto R, Maejima T, Kawamura T, Metoki H, Asayama K, Kikuya M, Ohkubo T, Kohzuki M, Takahashi K, Imai Y . Increased expression of (pro)renin receptor in the remnant kidneys of 5/6 nephrectomized rats. Regul Pept 2010; 159: 93–99.
Kaneshiro Y, Ichihara A, Sakoda M, Takemitsu T, Nabi AH, Uddin MN, Nakagawa T, Nishiyama A, Suzuki F, Inagami T, Itoh H . Slowly progressive, angiotensin II-independent glomerulosclerosis in human (pro)renin receptor-transgenic rats. J Am Soc Nephrol 2007; 18: 1789–1795.
Kobori H, Urushihara M, Xu JH, Berenson GS, Navar LG . Urinary angiotensinogen is correlated with blood pressure in men (Bogalusa Heart Study). J Hypertens 2010; 28: 1422–1428.
Kobori H, Alper AB Jr., Shenava R, Katsurada A, Saito T, Ohashi N, Urushihara M, Miyata K, Satou R, Hamm LL, Navar LG . Urinary angiotensinogen as a novel biomarker of the intrarenal renin-angiotensin system status in hypertensive patients. Hypertension 2009; 53: 344–350.
Takahashi K, Yamamoto H, Hirose T, Hiraishi K, Shoji I, Shibasaki A, Kato I, Kaneko K, Sasano H, Satoh F, Totsune K . Expression of (pro)renin receptor in human kidneys with end-stage kidney disease due to diabetic nephropathy. Peptides 2010; 31: 1405–1408.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Morimoto, S., Ando, T., Niiyama, M. et al. Serum soluble (pro)renin receptor levels in patients with essential hypertension. Hypertens Res 37, 642–648 (2014). https://doi.org/10.1038/hr.2014.46
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2014.46
Keywords
This article is cited by
-
The (pro)renin receptor as a pharmacological target in cardiorenal diseases
Hypertension Research (2023)
-
Sex differences in soluble prorenin receptor in patients with type 2 diabetes
Biology of Sex Differences (2021)
-
The evolving complexity of the collecting duct renin–angiotensin system in hypertension
Nature Reviews Nephrology (2021)
-
Serum soluble (pro)renin receptor level as a prognostic factor in patients undergoing maintenance hemodialysis
Scientific Reports (2021)
-
The (pro)renin receptor: a novel biomarker and potential therapeutic target for various cancers
Cell Communication and Signaling (2020)