Abstract
This study sought to determine if apocynin, a nicotinamide adenine dinucleotide phosphate oxidase inhibitor, would attenuate arterial stiffness in salt-sensitive hypertensive rats via structural and functional changes in conduit arteries. We showed that tail blood pressure was significantly higher in deoxycorticosterone acetate-salt-induced hypertensive (DSH) rats compared with the sham control group (P<0.01). Morphological analysis and biochemical assay showed that large arteries in DSH rats underwent significant remodeling including increased medial thickness in carotid arteries compared with the control rats (194.25±5.66 vs. 120.48±7.93 μm, P<0.05) and increased collagen deposition in thoracic aorta (1.03±0.09 vs. 0.85±0.04 mg cm−1, P<0.05). These changes were associated with increases in reactive oxygen species (ROS) level and increased thoracic aortic stiffness compared with the control rats (6.21±0.79 m s−1 vs. 4.64±0.59 m s−1, P<0.01). Treatment with apocynin significantly prevented ROS increases and collagen deposition (0.84±0.04 vs. 1.03±0.09 mg cm−1, P<0.05), and reduced arterial stiffness as shown by decreased pulse wave velocity in the thoracic aorta (5.31±0.88 vs. 6.21±0.79 m s−1, P<0.01). Additionally, apocynin prevented carotid artery wall thickening (58.57±3.40 vs. 78.89±4.10 μm, P<0.05). In conclusion we have shown that increased ROS level is associated with increased aortic stiffness, and deposition of collagen in the aortic arterial wall in DSH rats. Apocynin prevented ROS increases and arterial stiffness in DSH rats. Antioxidant therapy may be a potential treatment of large arterial stiffness in salt-sensitive hypertension.
Similar content being viewed by others
Introduction
Increasing evidence suggests that large artery stiffness (often measured by pulse wave velocity, PWV) predicts cardiovascular events.1 Stiffening of large central arteries, particularly the aorta, occurs during physiological processes such as aging and during pathological processes such as hypertension, diabetes mellitus and end-stage renal disease. It has also been shown that abnormal collagen deposition in the aortic arterial wall contributes to increased arterial stiffness.2, 3
Reactive oxygen species (ROS) have major roles in the initiation and progression of hypertension through various pathological mechanisms involved in vascular remodeling.4 Previous data have shown that ROS are involved in aging-related arterial stiffness.5 In addition, Delles et al.6 reported that there was a functional relationship between oxidative stress and vascular stiffness, and that strategy to reduce vascular oxidative stress benefited patients with high levels of vascular stiffness. In contrast, ROS were not responsible for arterial stiffness in a calcified model of hypertension.7, 8 Eskurza et al.7 reported that treatment of hypertensive patients with ascorbic acid for 30 days did not affect large elastic artery compliance and blood pressure (BP). The sympathetic activity and ambulatory BP in Africans study showed that large artery stiffness was not associated with ROS content in normotensive and hypertensive participants.9 However, the involvement of ROS in arterial stiffness and the effect of ROS inhibition on large artery stiffness remain largely unknown.
The major source of ROS in vascular cells is from the increased activity of an NADH/NADPH (nicotinamide adenine dinucleotide phosphate)-driven oxidase, which accounts for almost 60% of vascular ROS production.7, 8, 10, 11 Apocynin, a specific NADPH-oxidase inhibitor, interferes with p47phox and p67phox associations with the cell membrane components of the NADH oxidase complex, and has been extensively used in cardiovascular studies.12, 13, 14 Additionally, apocynin has been shown to improve endothelium-dependent vasodilation and NO production in isolated vessels.11, 15 We have recently demonstrated that apocynin induces dose-dependent vasodilatation.16 Apocynin-induced vascular superoxide production may be involved in vascular function, but whether apocynin attenuates arterial structural changes and improves aortic stiffness has yet to be determined.
Deoxycorticosterone acetate (DOCA)-salt hypertension (DSH) is a classic hypertensive model associated with markedly high levels of ROS and depressed plasma rennin activity, which can be used to study the effect of hypertension, in the absence of circulating angiotensin II, on vascular superoxide production and vascular reactivity.17 Studies have shown increased collagen deposition in rat aorta, indicating that large arterial stiffness may be increased.
In the present study, we hypothesized that aortic stiffness is increased in DSH rats, and that pharmacological interference with apocynin would attenuate arterial stiffness and corresponding changes in DSH rats.
Methods
Experimental animals
All experimental procedures were approved by the Animal Care and Use Committee, and were conducted under the guidelines for the care and use of laboratory animals as established by the Shanghai Institute of Hypertension (permit number: 2008125). DSH rats were generated as previously described.12 Briefly, DSH animals were anesthetized and uninephrectomized. After 7 days of recovery, rats were administered DOCA (D7000, Sigma, St Louis, MO, USA) s.c. at a dose of 12.5 mg per rat per week for 5 weeks and 1% NaCl was added to drinking water. The sham group received sham operation without kidney removal and given normal water. For the apocynin treatment groups, three concentrations of apocynin (0.5, 1.0 and 1.5 mM, Sigma) were administered orally in a tap water solution. Six weeks after surgery, central BP and PWV were measured as previously described.13
Aortic wall structure and composition
After delipidation and recording of dry weight, the thoracic aorta was subjected to hydrolysis in 6 M HCl for 16 h, and the resulting hydrolyzate was used in assays for hydroxyproline, desmosine and isodesmosine content. Briefly, hydroxyproline content was determined by the chloramine T and the paradimethylaminobenzaldehyde method.14 Collagen content was calculated as hydroxyproline content × 7.46. The content of the elastin-specific cross-linking amino acids desmosine and isodesmosine was determined by capillary zone electrophoresis and ultraviolet detection. Elastin content was calculated as (desmosine+isodesmosine) × 200. Elastin (or collagen) content is expressed as mg cm−1 of the aorta.
Ultrasound biomicroscopy (UBM), histological examination and immunocytochemistry, dihydroethidium fluorescence dye, lucigenin chemiluminescence and statistical analysis detailed descriptions are provided in the Supplementary Files (available online at http://www.nature.com/hr).
Results
Effect of apocynin on peripheral BP in DSH rats
There were no significant differences in baseline characteristics between groups before treatment (Table 1). The body weight of DSH rats was significantly lower compared with those in the sham group 6 weeks after the onset of experiments (P<0.01). Apocynin treatment had no effect on body weight of DSH rats.
BP was measured by tail-cuff method every 2 weeks. Systolic blood pressure (SBP) of all DSH rats increased gradually during the first 2 weeks of experiments, whereas sham group SBPs remained unchanged. At the end of the 6th week, SBP in DSH rats was significantly higher compared with SBP in the sham group (183.89±9.87 vs. 130.74±4.12 mm Hg, P<0.01). Treatment with 1 mM and 1.5 mM apocynin in DSH rats significantly lowered SBP compared with the sham group (159.83±7.99, 156.83±11.47mm Hg, respectively, P<0.05), while 0.5 mM apocynin treatment had no effect on SBP (177.06±5.70 mm Hg, P>0.05, Table 1).
Effect of apocynin on central BP in DSH rats
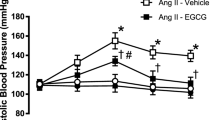
Peripheral BP does not always change in parallel with central BP. Central BP has been shown to be a stronger determinant of cardiovascular damage and complications in various clinical settings.18 Thus, we also measured central BP using a catheter placed into the descending aorta at the end of the experiment. Central SBP, central diastolic BP and central mean BP were all increased in the DSH group (Figure 1). No significant BP difference was observed after treatment with 0.5 mM apocynin. SBP in the 1 and 1.5 mM apocynin treatment groups decreased significantly compared with SBP in untreated DSH rats (135.36±8.05, 140.18±3.18 vs. 157.90±5.01 mm Hg, P<0.05). Diastolic BP was significantly reduced in the 1.0 and 1.5 mM apocynin groups compared with untreated DSH rats (107.15±30.44, 105.74±11.44 vs. 128.02±15.44 mm Hg, P<0.05).
Effect of apocynin on central BP in DSH rats. Central BP was measured by putting a catheter in the aortic root. Changes of central systolic BP (central SBP; a), central diastolic BP (central DBP; b), central mean BP (central MBP; c) and central pulse pressure (central PP; d) in sham control rats, DSH rats and DSH rats treated with 1.0 or 1.5 mM apocynin. *P<0.05 compared with DSH group; **P<0.01 compared with DSH group.
Effect of apocynin on thoracic aortic stiffness in DSH rats
To study the effect of apocynin on changes in aortic stiffness in DSH rats, we measured PWV invasively in the different groups. PWV was significantly higher in DSH rats compared with the sham group (6.21±0.79 vs. 4.66±0.59 m s−1, P<0.01, Figure 2). Treatment with 1.0 and 1.5 mM apocynin significantly decreased the PWV in DSH rats compared with the sham group (5.17±0.88, 5.31±0.88 m s−1, respectively, P<0.05). Treatment with 0.5 mM apocynin had no effect on PWV.
Effect of apocynin on structural changes in large arteries in DSH rats
To determine whether changes in aortic structure could be involved in the effect of apocynin, UBM of the carotid artery was conducted (Figure 3a). Intima-media thickness (IMT) was significantly higher in DSH rats compared with the sham group after 6 weeks (78.89±4.10 vs. 52.50±2.50 μm, P<0.01, Figure 3c). However, all dosages of apocynin treatment significantly ameliorated the increase in IMT (64.17±4.17, 65.00±4.47 and 58.57±3.40 μm, respectively, P<0.05, Figure 3c).
Effect of apocynin on IMT in common carotid arteries and aortic medial thickness in DSH rats. Representative ultrasound biomicroscopic images (a) and summarized data (c) showing the changes in IMT in the common carotid artery in sham control rats, DSH rats, and DSH rats treated with 1.0 or 1.5 mM apocynin. Representative hematoxylin and eosin staining images (b) and summarized data (d) showing the changes in medial thickness in thoracic aorta in sham control rats, DSH rats, and DSH rats treated with 1.0 or 1.5 mM apocynin. *P<0.05, **P<0.01 vs. DSH group. A full color version of this figure is available at the Hypertension Research journal online.
Morphological changes were observed in the five groups. Hematoxylin and eosin staining showed that the media of the aorta in DSH rats was significantly thicker compared with the sham group (194.25±5.66 vs. 120.48±7.93 μm, P<0.01, Figure 3b). Treatment with 1.0 and 1.5 mM apocynin induced a significant reduction in medial thickness compared with the untreated DSH group (164.25±10.16 and 165.52±11.22 μm, respectively, P<0.05, Figure 3d).
Effect of apocynin on aortic wall composition in DSH rats
To determine the relationship between ROS and arterial wall composition changes, we examined changes in the arterial extracellular matrix content in DSH rats. As shown in Table 2, the amount of collagen was increased in DSH rats compared with the sham group (1.03±0.09 vs. 0.85±0.04 mg cm−1, P<0.05). After treatment with 1.5 mM apocynin, the amount of collagen significantly decreased by 18% (0.84±0.04 mg cm−1, P<0.05). However, there was no change in the elastin content in any group, and no significant difference in the elastin: collagen ratio between groups.
The use of Masson trichrome staining is a valuable histopathology tool that shows fibrotic changes, particularly increases in the amount of collagen.16 Our data revealed marked increases in collagen deposition in the media and adventitia area in DSH rats compared with those in the control group (Figure 4). After apocynin treatment, the amount of collagen reduced gradually in a dose-dependent manner. However, there were no significant changes in the quantity and distribution of elastin in vessels from the different groups.
Effect of apocynin on collagen and elastin content in thoracic aorta in DSH rats. Representative images showing the changes of collagen in Masson staining (a) and elastin in Weigert staining (b) in thoracic aorta in sham control rats, DSH rats, and DSH rats treated with 1.0 or 1.5 mM apocynin. A full color version of this figure is available at the Hypertension Research journal online.
Effect of apocynin on ROS production and p47phox expression in arteries of DSH rats
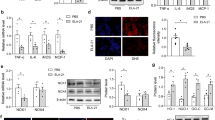
To determine whether the changes in aortic stiffness are mediated by apocynin-induced ROS decreases, the change in ROS content in the thoracic aorta was measured in the different groups. Dihydroethidium staining revealed that superoxide production in aorta sections was increased in DSH rats (Figure 5a). Lucigenin-enhanced chemiluminescence showed that ROS level was significantly increased in DSH rats compared with the SHAM group (P<0.01). Apocynin inhibited ROS production in a dose-dependent manner, but only 1.5 mM apocynin treatment significantly reduced ROS level (436.50±61.92 vs. 781.65±108.68 RLU min−1 mg−1, P<0.05). Apocynin treatment noticeably reduced ROS production in the thoracic aorta from DSH rats, as measured by dihydroethidium staining and lucigenin-enhanced chemiluminescence assay (Figure 5c). Apocynin also inhibited enhanced p47phox expression in DSH rat aortas (Figure 5b).
Effect of apocynin on superoxide anion production and p47phox expression in thoracic aorta in deoxycorticosterone acetate (DOCA)-salt hypertensive rats. Representative fluorescence microscopic images (a) showing the effect of apocynin on superoxide anion production by the oxidative fluorescent dye dihydroethidium in DSH rats. Representative photographs of immunostaining of p47phox in the aorta of rats (b). Brown dots indicate p47phox-positive cells. p47phox expression increased in the media area of DSH rats, which was suppressed by apocynin treatment. Lucigenin-enhanced chemiluminescence showed that 1.5 mM apocynin treatment significantly inhibited ROS production in DSH rats (c).Values are expressed as mg min−1 per dry weight. *P<0.05, **P<0.01 vs. DSH group.
Discussion
The cardiovascular remodeling in DSH rats includes fibrosis and hypertrophy in the heart and large arteries.19 In the present study, we found that remodeling of large arteries lead to severe arterial thickness, collagen deposition and increased arterial stiffness, associated with increases in the levels of ROS. In addition to its antihypertensive effect, the pharmacological NADPH-oxidase inhibitor, apocynin, blocked the progression of arterial stiffness in DSH rats and ameliorated the structural and compositional changes in the aorta. These results indicate that direct inhibition of the source of superoxide generation may improve arterial stiffness associated with hypertension.
NADPH oxidase, one of the most important sources of O2− in arteries, has been shown to be elevated in the aortic wall of DSH rats.20 In this study, we report for the first time that elevated aortic stiffness was associated with increased O2− generation in DSH rats, and that apocynin improved aortic stiffness after 6 weeks. Previous data have also shown that ROS are involved in aging-related stiffness.5 In contrast, ROS have been shown to not be involved in arterial stiffness in a calcified model of hypertension.7, 8 Overall, the results presented here and in previous studies indicate that the role of ROS in arterial stiffness may depend on the animal model used and vary in its significance as a contributory factor.
Several experimental studies have suggested the use of antioxidant treatment as an alternative strategy to prevent or reduce vascular complications associated with increased oxidative stress. For example, resveratrol, a constituent of red wine, has been shown to prevent adverse changes in the cardiovascular system in DSH rats.19 In humans, trans-resveratrol increased flow-mediated vasodilation in overweight/obese individuals with unmedicated borderline hypertension and increased cerebral blood flow and hemoglobin status in healthy young adults.21, 22 In addition, vitamins E, C and A have been shown to have antioxidant properties both in in vitro and in animal research studies.23 Although in most observational studies antioxidant vitamins have been shown to have beneficial effects for the cardiovascular system, data from multicenter clinical studies such as HOPE (Heart Outcomes Prevention and Evaluation Study) and HPS (Heart Prevention Study) have not shown a clinical benefit of antioxidant vitamin C or vitamin E treatment for cardiovascular events.24, 25 A possible explanation for this is that ROS have some important functions in intracellular redox signaling, whereas antioxidants are not able to entirely remove but keep these oxidants at a level below that which will trigger the inflammatory cascade.26, 27
From the results above, the understanding of the molecular nature of oxidative stress has been altered. Oxidative stress is no longer thought about as a simple imbalance between the production and scavenging of ROS, but a dysfunction of enzymes involved in ROS production, such as NADPH oxidase.28 However, most NADPH-oxidase inhibitors have weaknesses in bioavailability, efficacy and toxicity for their therapeutic use.29 Apocynin, a constituent of the Himalayan medicinal herb Picrorhiza kurroa, is regarded as an inhibitor of NADPH oxidase, but is quite different from the vitamin antioxidants.30, 31 Apocynin has been used as a traditional NADPH-oxidase inhibitor in many experimental models.32, 33 The mechanism of action of apocynin involves the impairment of the cytosolic subunit p47phox translocation to the cell membrane and, thus, failure to assemble the NADPH-oxidase complex.30, 31 It has been used as a liver and cardio-tonic in the treatment of jaundice and asthma.34, 35 It is a prodrug that metabolizes into dimers via the action of peroxidases.36 Apocynin has a very good safety profile (LD50: 9 g kg−1 upon oral administration in mice), and side effects have not been reported.29 After intragastric doses of 1 mmol kg−1 in rats, apocynin was rapidly excreted in the urine mainly unchanged, and the urinary excretion (48 h) was 97%.37 Recently, two in vivo studies indicated that inhaled apocynin markedly reduced O3−-induced hyperreactivity and H2O2 concentration in exhaled breath condensates, and was associated with a significant decrease in the concentration of NO3−.31, 38 Apocynin was well tolerated, and no adverse events were observed in these two studies.
Apocynin may also have some positive effects other than its inhibition of the subunits of NADPH oxidase. Some authors have questioned apocynin as a pure NADPH-oxidase inhibitor, because the formation of the apocynin dimer required the activity of myeloperoxidase, which is not expressed in vascular endothelial or smooth muscle cells.39 As a consequence, it has been thought that apocynin could act only as an antioxidant in vascular cells, independent of NADPH oxidase. However, in another study, Wang et al.40 indicated the bioavailability of apocynin is through its conversion to a glycoconjugate, not to diapocynin. Nevertheless, in animals or humans subjected to oxidative stress, apocynin has been demonstrated to reverse endothelial NO dysfunction.11 In one of our previous studies, apocynin-induced rat aorta relaxation in vascular smooth muscle cells occurred via the activation of voltage-gated potassium channels.16 Apocynin is also associated with some anti-inflammatory properties in the vasculature. Diabetes-induced increases in ICAM-1 expression and leukostasis in retinal vessels were significantly inhibited by apocynin treatment.41
In conclusion, the findings presented in this study suggest that apocynin is effective in preventing the progression of arterial stiffness in hypertension. Apocynin abolished ROS-induced arterial composition changes, especially collagen deposition associated with salt-sensitive hypertension. Further studies must focus on the mechanisms of apocynin metabolism, which may help to design novel therapeutic candidates for cardiovascular diseases.
References
Dao HH, Essalihi R, Graillon JF, Lariviere R, De Champlain J, Moreau P . Pharmacological prevention and regression of arterial remodeling in a rat model of isolated systolic hypertension. J Hypertens 2002; 20: 1597–1606.
Dellegrottaglie S, Sands RL, Gillespie BW, Gnanasekaran G, Zannad F, Sengstock D, Finkelstein F, Kiser M, Eisele G, Hinderliter AL, Levin NW, Cattan V, Saran R, Rajagopalan S . Association between markers of collagen turnover, arterial stiffness and left ventricular hypertrophy in chronic kidney disease (CKD): the Renal Research Institute (RRI)-CKD study. Nephrol Dial Transplant 2011; 26: 2891–2898.
Gabbiani F, Midtgaard J, Knopfel T . Synaptic integration in a model of cerebellar granule cells. J Neurophysiol 1994; 72: 999–1009.
Han WQ, Zhu DL, Wu LY, Chen QZ, Guo SJ, Gao PJ . N-acetylcysteine-induced vasodilation involves voltage-gated potassium channels in rat aorta. Life Sci 2009; 84: 732–737.
Lei Y, Yang J, Zhao H . Experimental study on extracts from ginseng, notoginseng and chuanxiong for delaying vascular aging in senescent mice. Zhongguo Zhong Xi Yi Jie He Za Zhi 2010; 30: 946–951.
Delles C, Zimmerli LU, McGrane DJ, Koh-Tan CH, Pathi VL, McKay AJ, Steedman T, Dargie HJ, Hamilton CA, Dominiczak AF . Vascular stiffness is related to superoxide generation in the vessel wall. J Hypertens 2008; 26: 946–955.
Eskurza I, Monahan KD, Robinson JA, Seals DR . Ascorbic acid does not affect large elastic artery compliance or central blood pressure in young and older men. Am J Physiol Heart Circ Physiol 2004; 286: H1528–1534.
Lalaoui MZ, El Midaoui A, de Champlain J, Moreau P . Is there a role for reactive oxygen species in arterial medial elastocalcinosis? Vascul Pharmacol 2007; 46: 201–206.
Kruger R, Schutte R, Huisman HW, Van Rooyen JM, Malan NT, Fourie CM, Louw R, van der Westhuizen FH, van Deventer CA, Malan L, Schutte AE . Associations between reactive oxygen species, blood pressure and arterial stiffness in black South Africans: the SABPA study. J Hum Hypertens 2012; 26: 91–7.
Touyz RM, Briones AM . Reactive oxygen species and vascular biology: implications in human hypertension. Hypertens Res 2011; 34: 5–14.
Hamilton CA, Brosnan MJ, Al-Benna S, Berg G, Dominiczak AF . NAD(P)H oxidase inhibition improves endothelial function in rat and human blood vessels. Hypertension 2002; 40: 755–762.
Ammarguellat F, Larouche I, Schiffrin EL . Myocardial fibrosis in DOCA-salt hypertensive rats: effect of endothelin ET(A) receptor antagonism. Circulation 2001; 103: 319–324.
Fitch RM, Vergona R, Sullivan ME, Wang YX . Nitric oxide synthase inhibition increases aortic stiffness measured by pulse wave velocity in rats. Cardiovasc Res 2001; 51: 351–358.
Marque V, Kieffer P, Atkinson J, Lartaud-Idjouadiene I . Elastic properties and composition of the aortic wall in old spontaneously hypertensive rats. Hypertension 1999; 34: 415–422.
Zhang R, Ran HH, Ma J, Bai YG, Lin LJ . NAD(P)H oxidase inhibiting with apocynin improved vascular reactivity in tail-suspended hindlimb unweighting rat. J Physiol Biochem 2012; 68: 99–105.
Han WQ, Wong WT, Tian XY, Huang Y, Wu LY, Zhu DL, Gao PJ . Contributory role of endothelium and voltage-gated potassium channels in apocynin-induced vasorelaxations. J Hypertens 2010; 28: 2102–2110.
Fenning A, Harrison G, Rose’meyer R, Hoey A, Brown L . l-Arginine attenuates cardiovascular impairment in DOCA-salt hypertensive rats. Am J Physiol Heart Circ Physiol 2005; 289: H1408–H1416.
Schillaci G, Grassi G . Central blood pressure: getting to the heart of the matter. J Hypertens 2010; 28: 237–239.
Chan V, Fenning A, Iyer A, Hoey A, Brown L . Resveratrol improves cardiovascular function in DOCA-salt hypertensive rats. Curr Pharma Biotechnol 2011; 12: 429–436.
Beswick RA, Dorrance AM, Leite R, Webb RC . NADH/NADPH oxidase and enhanced superoxide production in the mineralocorticoid hypertensive rat. Hypertension 2001; 38: 1107–1111.
Wong RH, Howe PR, Buckley JD, Coates AM, Kunz I, Berry NM . Acute resveratrol supplementation improves flow-mediated dilatation in overweight/obese individuals with mildly elevated blood pressure. Nutr Metab Cardiovasc Dis 2011; 21: 851–856.
Kennedy DO, Wightman EL, Reay JL, Lietz G, Okello EJ, Wilde A, Haskell CF . Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: a double-blind, placebo-controlled, crossover investigation. Am J Clin Nutr 2010; 91: 1590–1597.
Anderson D, Phillips BJ . Comparative in vitro and in vivo effects of antioxidants. Food chem toxicol 1999; 37: 1015–1025.
Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P . Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 2000; 342: 154–160.
Traber MG, Stevens JF . Vitamins C and E: beneficial effects from a mechanistic perspective. Free Radic Biol Med 2011; 51: 1000–1013.
Rhee SG . Cell signaling. H2O2, a necessary evil for cell signaling. Science 2006; 312: 1882–1883.
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J . Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007; 39: 44–84.
Schramm A, Matusik P, Osmenda G, Guzik TJ . Targeting NADPH oxidases in vascular pharmacology. Vascul Pharmacol 2012; 56: 216–231.
Yu J, Weiwer M, Linhardt RJ, Dordick JS . The role of the methoxyphenol apocynin, a vascular NADPH oxidase inhibitor, as a chemopreventative agent in the potential treatment of cardiovascular diseases. Curr vasc pharmacol 2008; 6: 204–217.
Barbieri SS, Cavalca V, Eligini S, Brambilla M, Caiani A, Tremoli E, Colli S . Apocynin prevents cyclooxygenase 2 expression in human monocytes through NADPH oxidase and glutathione redox-dependent mechanisms. Free Radic Biol Med 2004; 37: 156–165.
Peters EA, Hiltermann JT, Stolk J . Effect of apocynin on ozone-induced airway hyperresponsiveness to methacholine in asthmatics. Free Radic Biol Med 2001; 31: 1442–1447.
Lafeber FP, Beukelman CJ, van den Worm E, van Roy JL, Vianen ME, van Roon JA, van Dijk H, Apocynin Bijlsma JW . a plant-derived, cartilage-saving drug, might be useful in the treatment of rheumatoid arthritis. Rheumatology 1999; 38: 1088–1093.
Zhang Y, Chan MM, Andrews MC, Mori TA, Croft KD, McKenzie KU, Schyvens CG, Whitworth JA . Apocynin but not allopurinol prevents and reverses adrenocorticotropic hormone-induced hypertension in the rat. Am J Hypertens 2005; 18: 910–916.
Simons JM, Hart BA, Ip Vai Ching TR, Van Dijk H, Labadie RP . Metabolic activation of natural phenols into selective oxidative burst agonists by activated human neutrophils. Free Radic Biol Med 1990; 8: 251–258.
Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ . Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol 1994; 11: 95–102.
Ximenes VF, Kanegae MP, Rissato SR, Galhiane MS . The oxidation of apocynin catalyzed by myeloperoxidase: proposal for NADPH oxidase inhibition. Arch Biochem Biophys 2007; 457: 134–141.
Adams NL, Barlow A, Hiddlestone J . Obtaining ergonomics information about industrial injuries: A five-year analysis. Appl Ergon 1981; 12: 71–81.
Stefanska J, Sokolowska M, Sarniak A, Wlodarczyk A, Doniec Z, Nowak D, Pawliczak R . Apocynin decreases hydrogen peroxide and nitrate concentrations in exhaled breath in healthy subjects. Pulm Pharmacol Ther 2010; 23: 48–54.
Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP . Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 2008; 51: 211–217.
Wang Q, Smith RE, Luchtefeld R, Sun AY, Simonyi A, Luo R, Sun GY . Bioavailability of apocynin through its conversion to glycoconjugate but not to diapocynin. Phytomedicine 2008; 15: 496–503.
Al-Shabrawey M, Rojas M, Sanders T, Behzadian A, El-Remessy A, Bartoli M, Parpia AK, Liou G, Caldwell RB . Role of NADPH oxidase in retinal vascular inflammation. Invest Ophthalmol Vis Sci 2008; 49: 3239–3244.
Acknowledgements
This study was supported financially by grants from the National Basic Research Program of China (2011CB503905), the National High-Tech Research and Development program (2012AA02A516), the National Natural Science Foundation of China (81070261), and Oriental Scholar (Chair professor).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Hypertension Research website
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, QZ., Han, WQ., Chen, J. et al. Anti-stiffness effect of apocynin in deoxycorticosterone acetate-salt hypertensive rats via inhibition of oxidative stress. Hypertens Res 36, 306–312 (2013). https://doi.org/10.1038/hr.2012.170
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2012.170
Keywords
This article is cited by
-
Tributyltin chloride disrupts aortic vascular reactivity and increases reactive oxygen species production in female rats
Environmental Science and Pollution Research (2017)
-
Renal tubulointerstitial damage and salt-sensitive hypertension in chronic kidney disease: is the tubulointerstitium relevant beyond the glomerulus?
Hypertension Research (2015)