Abstract
Hypertension is the most prevalent chronic disease World-wide, and the leading preventable risk factor for cardiovascular disease (CVD). Few patients accomplish the objective of decreasing blood pressure and avoiding hypertensive target organ damage after treatments with antihypertensive agents which opens the door for other treatments, such as herbal-and antihypertensive combination therapy. Captopril (CAP), as a-pril which inhibits angiotensin converting enzyme has long been used in the management of hypertension and CVD. Gedan Jiangya Decoction (GJD) is known for antihypertensive effects in prior studies. The research is aimed to determine whether GJD in combination with captopril has antihypertensive, kidney protective, antioxidant, and vasoactive effects in spontaneously hypertensive rats (SHR). Regular measurements of systolic and diastolic blood pressure (SBP and DBP), and body weight were monitored weekly. H&E staining was utilized to examine histopathology. The combined effects were studied using ELISA, immunohistochemistry, and qRT-PCR. Significant reductions in SBP, DBP, aortic wall thickness, and improvement in renal tissue were observed following GJD + CAP treatment, with increased serum levels of NO, SOD, GSH-Px, and CAT and decreases in Ang II, ET-1, and MDA. Similarly, GJD + CAP treatment of SHR's significantly decreased ET-1 and AGTR1 mRNA and protein expression while increasing eNOS mRNA and protein expression in thoracic aorta and kidney tissue. In conclusion, the present investigation found that GJD + CAP treatment decreases SHR blood pressure, improves aorta remodeling and renal protection, and that this effect could be attributable, in part, due to antioxidant and vascular tone improvement.
Similar content being viewed by others
Introduction
Hypertension is among the main preventable risk factors for premature morbidity and mortality among humans in both the developing and developed worlds1,2,3. A study of 1,100,507 participants in low- and middle-income countries found that 192,441 (17.5%) had high blood pressure; 39.2% were diagnosed with hypertension, 29.9% were treated, and only 10.3% had hypertension under control4. In high-income countries, the rate of hypertension control is less than 25%5, whereas in low- and middle-income countries, it is only 8%6. Prolonged hypertensive state can cause hypertension-related target organ damage, which results in structural and functional impairment of critical internal organs such as the heart, kidneys, and brain7. These organs are primary targets of hypertension since they receive the majority of the blood flowing through the vascular system8. Of many mechanisms, reactive oxygen species (ROS) are produced primarily during hypertension development, by endothelial nitric oxide (eNOS) uncoupled to produce superoxide anion due to a combined reaction between reactive oxygen species and vasoconstriction factors such as endothelin-1 (ET-1) and angiotensin II (Ang II)9. The primary marker of hypertension is vascular damage and the etiology of hypertension-related target organ damage is the reduction in vasodilator nitric oxide and an elevation of oxidative stress, and vasoconstrictor factors ET-1 and Ang II10,11,12. Therefore, effective blood pressure-lowering intervention, along with antioxidants and a balance of vasoactive substances, has the potential for therapeutic intervention for preventing and treating hypertension-related cardiovascular disease10,12,13.
The development of hypertension is strongly influenced by various factors, including obesity, insulin resistance, high sodium intake, family history, and a sedentary lifestyle14. In the treatment of hypertension, there are multiple classes of drugs available. It is recommended to prioritize medications that have demonstrated effectiveness in reducing the risk of cardiovascular disease. Therefore, commonly used medications for hypertension treatment include angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), calcium channel blockers (CCBs), and thiazide diuretics15. Captopril, an ACE inhibitor, is widely utilized and has been proven to provide rapid antihypertensive effects, reduce peripheral vascular resistance, and offer protection against target organ damage associated with hypertension16,17.
In recent times, there has been growing evidence suggesting that Chinese herbal medicines can be effective in treating hypertension while maintaining a good safety profile18,19. Gedan Jiangya Decoction (GJD), a Chinese herbal formula (Patent Published No. CN114246896A) consists of six medicinal herbs, including Achyranthes bidentata Blume, Prunella vulgaris, Eucommia ulmoides Oliv, Pueraria lobata Ohwi, Salvia miltiorrhiza, and Uncaria rhynchophylla. Earlier studies have indicated that GJD (Chinese herbal medicine) can effectively reduce blood pressure in rats subjected to a high-salt diet. This blood pressure-lowering effect may be attributed to the decreased activation of the renin-angiotensin system20. In addition, our recently reported study revealed that the GJD could modulate cardiovascular inflammation and decreases blood pressure via regulating the NF-κB signal pathway in the spontaneously hypertensive rat21.
In the last decade, many studies have found that few patients have achieved the therapeutic goal of decreasing blood pressure from antihypertensive drugs and that herbal medicine (such as Chinese medicine) in combination therapy with antihypertensive drugs, is beneficial in decreasing blood pressure and preventing hypertensive related cardiovascular and kidney disease22,23, and the combination treatment has better antihypertensive efficacy than of monotherapy as it adds many mechanisms of action that block the different pathway of elevated blood pressure, in addition to offering greater protection to target organs than monotherapy and a lower risk of adverse effects23,24,25. The present study was designed to investigate the impact of GJD combined with captopril on blood pressure, oxidative stress, thoracic aorta remodeling, and kidney protection in spontaneously hypertensive rats.
Results
Arterial pressure measurement and body weight determination
From (Fig. 1A,B), SBP and DBP were significantly higher in the SHR group (191.19 and 142.63 mmHg) than in the age-matched WKY group (123.55 and 85.51 mmHg) (all P < 0.001). Compared with the SHR group, significant decreases in SBP and DBP were observed in rats after 6 weeks of treatment with GJD (143.49 and 98.88 mmHg) and CAP (148.10 and 103.11 mmHg) (all P < 0.001), whereas there was no large difference between these groups. Similarly, SBP and DBP decreased significantly in the GJD + CAP group than in the SHR group (136.19 and 90.26 mmHg) (all P < 0.001). In addition, rats receiving GJD + CAP had the most significant reduction in SBP and DBP compared with the CAP group (all P < 0.001) (Fig. 1A,B). Throughout the observation period, our data showed no statistically significant differences (P > 0.05) in body weight between groups (Fig. 1C). (All data in Supplementary file 1).
GJD and captopril combined effect on vasoconstriction factors Ang II, and ET-1 levels in serum
After 6 weeks of treatment, the SHR group had higher levels of the vasoconstrictor Ang II and ET-1 (625.3 and 125.3 ng/L) than the WKY group (246.6 and 47.8 ng/L) (all P < 0.001), whereas the levels of Ang II and ET-1 were significantly lower in the GJD, CAP, and GJD + CAP groups (Ang II 373.9, 406.9, and 290.5 ng/L; ET-1 65.6, 80.4, and 55.8 ng/L) than in the SHR group (all P < 0.001). In addition, the GJD + CAP group had significantly lower levels of Ang II and ET-1 than the CAP group (P = 0.01 and P = 0.03). (Fig. 2A,B).
Expression of AGTR1, ET-1 and eNOS in thoracic aorta by Immunohistochemistry and qRT-PCR
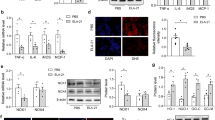
In the aorta, the immunohistochemical results are presented here as integrated optical density (IOD) values. AGTR1 and ET-1 expressions were significantly higher in the SHR group (49109 and 7656.41) than in the WKY group (20139 and 2290.94); meanwhile, eNOS expression was significantly reduced in the SHR group compared with the WKY group (12126.2 and 26101) (all P < 0.001), Fig. 3A–C. In contrast to the SHR group, the AGTR1 and ET-1 expression markedly decreased in GJD, CAP, and GJD + CAP groups (AGTR1 27885, 31036, and 21620; ET-1 3796.29, 4854.08, and 2924.48) (all P < 0.001), whereas eNOS expression dramatically increased in GJD, CAP, and GJD + CAP groups (19376.3, 17452.6, and 22689.3) (P = 0.002, P = 0.04, and P < 0.001), Fig. 3A–C. The GJD + CAP group demonstrated a significant reduction in AGTR1 and ET-1 and a substantial elevation in eNOS level than the CAP group (P = 0.02, P = 0.01, and P = 0.04).
Immunohistochemistry and mRNA expression of AGTR1, ET-1, and eNOS in the thoracic aorta. (A) protein expressions of AGTR1, (B) protein expressions of ET-1, (C) protein expressions of eNOS, (D) mRNA expression of AGTR1, (E) mRNA expression of ET-1, and (F) mRNA expression of eNOS. (× 200 Magnification, scale bar: 100 μm). The results are reported as means ± standard deviations. ***P < 0.001 versus WKY, #P < 0.05, ##P < 0.01, ###P < 0.001 versus SHR, and ΔP < 0.05 versus CAP groups. (n = 8).
The mRNA of AGTR1, ET-1, and eNOS was also examined using qRT-PCR. As shown in Fig. 3D–F, the SHR group's AGTR1 and ET-1 mRNA expression levels (3.21 and 5.24) increased dramatically more than the WKY group (1.00 and 1.00); meanwhile, eNOS mRNA expression level in SHR dramatically reduce more than WKY group (0.30 and 1.00) (all P < 0.001). The AGTR1 mRNA level was considerably lower in GJD, CAP, and GJD + CAP groups (1.72, 2.25, and 1.46) as compared to the SHR group (all P < 0.001), and the level of ET-1 mRNA was also dramatically reduced in GJD, CAP, and GJD + CAP groups (3.24, 3.80, and 2.54) (P < 0.001, P = 0.02, and P < 0.001), while the eNOS mRNA level significantly increased in GJD, CAP, and GJD + CAP groups (0.84, 0.59, and 1.04) (P < 0.001, P = 0.006, and P < 0.001). From Fig. 3D–F, the GJD + CAP group demonstrated a significant decrease in AGTR1 and ET-1 mRNA and a marked increase in eNOS mRNA level compared with the CAP group (P = 0.008, P = 0.05, and P < 0.001).
Expression of AGTR1, ET-1 and eNOS in kidney by Immunohistochemistry and qRT-PCR
As demonstrated in Fig. 4A–C, the IOD values of AGTR1 and ET-1 expression in the kidney were significantly higher in the SHR group (385676 and 195664) than in the WKY group (203427.6 and 117899), whereas the expression of eNOS was significantly lower in SHR group compared to that of the WKY group (128498 and 226023) (all P < 0.001). As shown in Fig. 4A–C that when compared to the SHR group, AGTR1 (240698.13, 297121.87, and 207926) (P < 0.001, P = 0.03, and P < 0.001) and ET-1(146094, 162293, and 131873) (P < 0.001, P = 0.01, and P < 0.001) expressions were dramatically reduced in GJD, CAP, and GJD + CAP groups and expression of eNOS was significantly increased in GJD, CAP, and GJD + CAP groups (179001, 168597, and 207052) (P = 0.002, P = 0.02, and P < 0.001). Figure 4D–F, both AGTR1 and ET-1 expressions were significantly lower, and eNOS was significantly higher in the GJD + CAP group versus the CAP group (all P = 0.03).
Immunohistochemistry and mRNA expression of AGTR1, ET-1, and eNOS in the kidney. (A) protein expressions of AGTR1, (B) protein expressions of ET-1, (C) protein expressions of eNOS, (D) mRNA expression of AGTR1, (E) mRNA expression of ET-1, and (F) mRNA expression of eNOS. (× 200 Magnification, scale bar: 100 μm). The results are reported as means ± standard deviations. ***P < 0.001 versus WKY, #P < 0.05, ##P < 0.01, ###P < 0.001 versus SHR, and ΔP < 0.05 versus CAP groups. (n = 8).
The AGTR1, ET-1, and eNOS mRNA expressions were also determined using qRT-PCR. The AGTR1 and ET-1 mRNA levels were considerably higher in the SHR group (7.12 and 4.01) than in the WKY group (1.00 and 1.00); however, the eNOS mRNA level in SHR was significantly lower than that in the WKY group (0.31 and 1.00) (all P < 0.001). From Fig. 4D, the AGTR1 mRNA level was significantly reduced in GJD, CAP, and GJD + CAP groups (3.34, 4.79, and 2.67) (all P < 0.001) following treatment compared with the SHR group. The ET-1 level was considerably lower in GJD, CAP, and GJD + CAP groups (3.18, 3.21, and 2.34) than in the SHR group (P = 0.02, P = 0.03, and P < 0.001), Fig. 4E. In contrast to the SHR group, the mRNA expression of eNOS in GJD, CAP, and GJD + CAP groups was significantly increased (0.77, 0.63, and 1.03) (P < 0.001, P = 0.005, and P < 0.001), Fig. 4F. As shown in Fig. 4D–F, The GJD + CAP group showed markedly lower AGTR1 and ET-1 mRNA expression and substantially higher eNOS mRNA expression than the CAP group (P = 0.001, P = 0.02, and P < 0.001).
GJD and captopril combined effect on oxidative stress
As shown in Fig. 5A–E, SHR had significantly lower levels of nitric oxide (NO) (2.2 µmol/L), superoxide dismutases (SOD) (143.4 U/ml), glutathione peroxidase (GSH-Px) (1054.6 U/ml), and catalase (CAT) (3.83 + 1 U/ml) and a significantly greater level of MDA (7.57 nmol/ml) than the age-matched WKY (5.47 µmol/L, 302.4 U/ml, 1736.3 U/ml, 8.64 U/ml, and 3.40 nmol/ml, respectively) (all P < 0.001). After 6 weeks of gavage treatment, NO level was significantly elevated in GJD, CAP, and GJD + CAP groups (4.01 µmol/L, 3.76 µmol/L, and 5.14 µmol/L) in comparison to SHR (P = 0.003, P = 0.01, and P < 0.001), Fig. 5A. The SOD serum level was significantly increased in GJD, CAP, and GJD + CAP groups (234.1, 214.9, and 284.0 U/ml) than that of the SHR group (P = 0.005, P = 0.04, and P < 0.001), Fig. 5B. In comparison to the SHR, the serum level of GSH-Px was also significantly improved in GJD, CAP, and GJD + CAP groups (1411.9, 1295.7, and 1518.9 U/ml) (P < 0.001, P = 0.01, and P < 0.001), Fig. 5C. After 6 weeks of treatment, the GJD, CAP, and GJD + CAP groups all had significantly greater CAT levels (6.59, 5.93, and 7.89 U/ml) compared to the SHR group (P < 0.001, P = 0.01, and P < 0.001), Fig. 5D. In contrast, the malondialdehyde (MDA) level in the rat's serum was reduced markedly in GJD, CAP, and GJD + CAP groups (4.90, 5.51, and 3.55 nmol/ml) in comparison to SHR (P = 0.002, P = 0.02, and P < 0.001), Fig. 5E. In contrast to the CAP group, the maximum increases in NO, SOD, GSH-Px, CAT, and minimum decreases in MDA, were observed in the rat's serum in the GJD + CAP group (P = 0.03, P = 0.05, P = 0.03, P = 0.02 and P = 0.03), Fig. 5A–E.
Biochemicals parameters to assess liver and kidney function
As markers of hepatic and renal function, AST (P = 0.4073), ALT (P = 0.9552), TP (P = 0.085), BUN (P = 0.0273), CRE (P = 0.9699), DBIL (P = 0.0795), GGT (P = 0.6133), and TBA (P = 0.9348). The results of the one-way analysis ANOVA revealed that there was no significant difference in all liver and kidney function indices except BUN. However, Tukey's multiple comparison test showed that there was no significant difference in all liver and kidney function indices of each group in pairs (Table 1).
HE staining results
SHR displayed aorta thickening (0.190 mm), as shown by an increase in wall thickness when compared to age-matched WKY rats (0.118 mm) (P < 0.001). According to measurements of the thoracic aorta thickness, the progression of aortic hypertrophy was significantly attenuated in GJD, CAP, and GJD + CAP groups (0.134, 0.142, and 0.124 mm) than the SHR group (all P < 0.001), and thoracic aorta thickness was dramatically reduced in the GJD + CAP group than the CAP group (P < 0.001), Fig. 6A,D. The kidney tissue showed mild congestive dilation of glomerular capillaries in the model group, and no obvious abnormality was found in the other groups (The tissue staining was uniform, the glomerular morphology and structure were normal, the tubular epithelial cells were closely arranged, and no obvious inflammation was found), Fig. 6B. Mild hepatocyte degeneration was seen in the liver tissue of the model group, and no obvious abnormality was found in the other groups (The tissue in the visual field was evenly stained, the hepatic cords were neatly arranged, the cytoplasm of the hepatocytes was abundant, and the morphology and structure were normal), Fig. 6C.
HE staining of thoracic aorta, kidney, and liver. (A) Thoracic aorta, (B) Kidney, (C) Liver, (D) Thoracic aorta thickness. (×200 Magnification, scale bar: 100 μm). The results are reported as means ± standard deviations. ***P < 0.001 versus WKY, ###P < 0.001 versus SHR, ΔP < 0.05 versus CAP groups. (n = 8).
Discussion
Hypertension is the leading preventable risk factor for CVD, which often needs the use of long-term antihypertensive medication therapy to manage blood pressure within the recommended therapeutic range1,26. Most antihypertensive drugs still provide inadequate blood pressure control, with just 39% of hypertension patients meeting the therapeutic objective of blood pressure less than 140/90 mmHg27, so a more effective and safe strategy using antihypertensive drug combinations with western medicine are needed. In China, combining Chinese and western medicine is a common treatment regimen for treating a wide range of diseases28. The primary idea for treating hypertension in the guide to prevent and treat hypertension is combination treatment, which is thought to be useful for strengthening the antihypertensive impact without increasing undesirable effects28. The systematic review found that combining Chinese herbal medicine with western medicine reduced SBP and DBP to the target level in primary hypertensive patients better than western medicine alone, and no patients discontinued therapy or withdrew due to serious adverse effects, suggesting that combining the Chinese herbal medicine with western medication may be safe for hypertension treatment29. Captopril, an angiotensin-converting enzyme inhibitor, is extensively used in the treatment of hypertension and cardiovascular disease, and it also possesses antioxidant properties30. Previous research found that GJD may lower systolic and diastolic blood pressure in high salt fed rats20. In this study, GJD was used in conjunction with western medicine to treat spontaneously hypertensive rats to assess antihypertensive, vasoactive effects, anti-oxidant, aorta remodeling, and kidney protection. We found that 6 weeks of GJD administration combined with captopril treatment could significantly decrease both SBP and DBP better than captopril alone or GJD alone. Thus, our results revealed that GJD in combination with captopril can control blood pressure efficiently in SHRs. This result corresponds to the finding of several previous studies where combination therapy especially Chinese herbal formula with captopril a western medicine has a significant effect in decreasing blood pressure31,32.
Ang II has previously been shown to be significant in the control of vascular tone. It binds to the Ang II receptor type 1 (AGTR1) and works as a powerful vasoconstrictor, modulates aldosterone production, and remodels the heart and arteries, ultimately leading to hypertension and cardiac dysfunction33. Furthermore, hypertension and associated cardiovascular problems are caused by aberrant activation of the renin-angiotensin system (RAS). Similar to previous findings, SHR expresses various major RAS-related genes in the kidneys34 and has increased serum Ang II levels35. Previous studies indicated that the GJD might decrease Ang II level and AGTR1 expression20,21. According to our investigation, serum Ang II levels and AGTR1 protein expression in the thoracic aorta and kidney tissue were considerably lowered in all treatment groups, with the combination group experiencing the most profound reductions.
Endothelial cells are key elements of blood arteries that regulate blood fluidity and fibrinolysis, vascular tone, angiogenesis, monocyte/leukocyte adhesion, and platelet aggregation36. ET-1, a powerful vasoactive substance, plays an important role in controlling endothelial function and has a significant endogenous biological vasoconstrictive action36,37. The previous finding indicated that the GJD might affect the ET-1 level in hypertensive rats' serum21, and captopril decreased the ET-1 level in the kidney of SHR rats31. These results were in line with previous studies where the vasoconstrictor factor ET-1 level in serum and ET-1 expression in kidney and thoracic aorta was markedly decreased throughout all treatment groups, with the GJD + CAP group experiencing the greatest reductions.
Oxidative stress is a common cause of damage in many disease states, and it arises when the body's production of ROS exceeds its antioxidant defenses38. Since ROS and redox signaling play crucial roles in cardiovascular disease and renal impairments, oxidative stress is particularly relevant in hypertension39. Multiple studies have shown higher levels of ROS in primary hypertensive individuals and diverse animal models of this disease40. The fact that these patients and animals have a reduced antioxidant level supports the idea that enhanced vascular oxidative stress may have a role in the pathophysiology of primary hypertension41,42,43,44. In hypertension, ROS are produced by NADPH oxidase, xanthine oxidase, uncoupled eNOS, and mitochondria45, as well as Ang II and ET-1, which trigger transcription factors and redox-sensitive signaling pathways that alter vasculature function and structure46. Enzymatic antioxidants include SOD, GSH-Px, and CAT47. These antioxidants protect cellular lipids, proteins, and DNA from oxidative damage48. In our study, SOD activity was significantly lower in SHR hypertensive rats compared to WKY rats in the control group. These results are consistent with earlier research12. SOD is one of the most important antioxidant enzymes that convert the harmful superoxide anion O2 − %to harmless oxygen and hydrogen peroxide49. The toxicity of superoxide is reduced during this reaction and no free radicals are created50. In contrast to the SHR group, treatment groups receiving GJD, CAP, and GJD + CAP in this study showed a significant increase in SOD. A significant increase was also seen in the GJD + CAP group.
CAT and GSH-Px are two other antioxidant enzymes that detoxify free radicals by degrading H2O2 to oxygen and water47. GSH-Px may also diminish lipid peroxides and other organic hydroperoxides, which are very cytotoxic47,51,52. In the current research, the activity of CAT and GSH-Px enzymes decreased more significantly in the SHR group than in the WKY group, whereas it increased markedly in the GJD, CAP, and GJD + CAP groups compared to the SHR. The GJD + CAP group showed the most significant increase in CAT and GSH-Px levels. The SHR group in our research revealed a reduction in the production of antioxidative enzyme CAT and GSH-Px, which might be caused when the oxidative load exceeds the defense capability53. It is well known that antioxidant treatment increases CAT and GSH-Px activity, which was validated in the current investigation, with a more substantial increase reported in the combination group.
Furthermore, MDA is an oxidative product formed in the body by free radicals destroying fat, which may be utilized as an oxidative damage marker54. The findings of this investigation revealed that SHR rats have significantly increased levels of MDA than the WKY control group, which correlates to the findings of previous studies on oxidative stress in hypertensive rats12,55. As a result, rising MDA levels in hypertension indicate an increase in lipid peroxidation. In the current investigation, treatment of SHR rats with GJD, CAP, and the combination group GJD + CAP reduced MDA levels significantly, with the combination group showing the greatest decrease. This effect may be due to the GJD and captopril that neutralize the free radicals generated by lipid peroxidation56,57. Consequently, a decrease in MDA levels in all treatment groups and a greater reduction in the combination group may be attributable to the antioxidant impact of the combination GJD with captopril.
Nitric oxide is a transmitter produced by eNOS that plays an important role in blood vessel vasodilation58,59. NO bioavailability may decrease during hypertension due to decreased eNOS production or increased NO inactivation by oxidative stress60. Indeed, several studies indicate that oxidative stress is responsible for decreased NO bioavailability, which leads to reduced vasodilation and endothelial dysfunction in individuals with hypertension61,62,63. Furthermore, NO is required to regulate renal perfusion and glomerular filtration in the kidneys60. Consequently, Ang II induced oxidative stress reduces NO bioavailability in the renal microvasculature, leading to increased afferent arteriolar tone and hypertension64,65. In our study, the SHR group had considerably lower levels of NO and eNOS than the WKY control group. It has also been observed that endothelial dysfunction in SHR has been linked to elevated reactive oxygen species (ROS) generation in the vascular endothelium, notably superoxide anions, which are known to inactivate NO and thereby reduce NO bioavailability66. Indeed, ROS may oxidize eNOS cofactors such as tetrahydrobiopterin, causing the enzyme to switch from dimeric to monomeric form67. In the monomeric state, eNOS is uncoupled and superoxide anion is generated instead of NO, which may have negative repercussions and contribute to cardiovascular disorders, including hypertension67. In our study, NO serum level and eNOS expression in the aorta and kidney tissues increased significantly following treatment with GJD, CAP, and the combination group GJD + CAP, with the combination group showing the greatest increase, perhaps due to its strong anti-oxidative impact.
Increasing artery wall thickness is a common feature of hypertension-induced widespread vascular remodeling68. The previous study confirmed that GJD reduces smooth muscle layer thickness21, In the current study, we found that GJD combined with captopril treatment more significantly reduced vessel wall thickness.
The evaluation of biochemical markers reveals that medicinal herbs may have a negative impact on renal and hepatic function. Various biochemical indicators are important in detecting liver impairment and renal dysfunction of which ALT and AST are common liver enzymes that may be used to detect changes in liver function69, and screening tests for renal functioning are urea and creatinine70, and serum total protein analysis provides an estimate of nutritional status as well as a diagnostic assessment for changes in renal functioning71. Renal dysfunction causes inefficient urea and creatinine excretion, resulting in their buildup in the blood. Furthermore, histopathology of the kidney and liver tissues is regarded as an important technique for demonstrating hazardous substance-induced liver and kidney damage72. In this study, the kidney morphology changed significantly in SHR, however, the renal tissue damage biomarkers CREA, and TP did not change significantly. These findings were consistent with previous research16, which found that morphology changes in SHR rats were significantly presented at 12 weeks, but did not show a significant change in the CREA, BUN, and TP biomarkers until week 20, indicating that new sensitive biomarkers are required to detect early renal damage rather than old ones. In the current research, the histological investigation revealed modest congestive dilatation of glomerular capillaries in the SHR group, but the morphology and structure of the glomeruli were normal in all other groups. The previous research demonstrated that SHR develops congestive dilatation of glomerular capillaries, glomerular hypertension, ischemia with arteriolar constriction, and glomerular sclerosis and these changes begin to reverse following therapy73, this was validated in the current study. Therefore, according to our results, GJD extracts alone or in combination with captopril did not create liver or kidney toxicity in healthy rats, and it did not produce negative and/or toxic effects through histological and biochemical examination. This suggests that the drug's prescription is safe and has a protective effect on the kidney. However, more studies are necessary to clarify these results.
The results of the current study indicated that combination therapy by GJD + Captopril decreased blood pressure and the possible mechanism is via improving the serum, kidney, and thoracic aorta vasoactive factors by decreasing the vasoconstriction ET-1 and AngII and AGTR1 and the possible mechanism due to that the GJD effect20,21 with captopril31,74 which decrease these vasoconstriction factors. The increase in the vasodilation NO and eNOS may be due to the antioxidant effect of the GJD formula combined with the anti-oxidant effect of captopril that has a free radical scavenger activity30,75,76 that increases eNOS expression and reverses its uncoupling state and more synthesis of NO, finally, decrease blood pressure and tissue protection. Together, the combination of GJD with captopril was found to have a significant decrease in blood pressure, an anti-oxidant effect, and an influence on vasoactive factors that may improve aorta remodeling and kidney protection. These findings offer preliminary evidence for the possible future therapeutic use of GJD in combination with captopril as antihypertensive and renal protective effects.
In conclusion, the combination of GJD and captopril was found to significantly lower blood pressure, improve aorta remodeling, and kidney protection, possibly through an anti-oxidant effect (as measured by a decrease in MDA and an increase in SOD, GSH-Px, and CAT) and an influence on vasoactive factors (as measured by an increase in NO and eNOS and a reduction in ET-1, Ang II, and AGTR1). This in vivo study provides basic evidence for the potential future therapeutic use of GJD in combination with captopril as an antihypertensive.
Materials and methods
Materials
Preparation of GJD
The GJD, an aqueous ethanol extract containing six herbal medications (as presented in Table 2) were divided into two parts and soaked for half an hour in ethanol solution in a 1 g:10 ml ratio; part one (Salvia miltiorrhiza 25 g, Pueraria lobate 30 g, Achyranthes bidentata 20 g, Eucommia ulmoides 15 g, and Prunella vulgaris 15 g were decocted twice for one and a half hours in 60% ethanol, (during reflux, maintain the solution's boiling)) and part two (Uncaria rhynchophylla 10 g was mixed with 70% ethanol and decocted two times for two hours at 65 ~ 75 °C). Degreasing gauze was layered six times for each part. After that, soaked, reheated, and filtered the solution, then combined the two filtrates. The ethanol was then distilled at low pressure that used a rotary evaporator, followed by drying under low pressure and vacuum to give extract powder of the two parts. Finally, the two extract powder parts were mixed to produce GJD. High performance liquid chromatography (HPLC) was established to determine the active ingredients and it mainly contained puerarin (Purity: 2.31%), tanshinone IIA (Purity: 0.25%), daidzein (Purity: 0.5%), ursolic acid (Purity: 0.6%), and cryptotanshinone (Purity: 0.26%)20. The GJD botanical herbs dosage preparation processes follow the patent published (No. CN114246896A) and previously published articles20,21.
Animal
The Vital River Laboratory Animal Technology Co. Ltd (Beijing, China) provided 32 SHR and 8 Wistar Kyoto (WKY) male rats aged 10 weeks (243 ± 10 g). License for experimental animals: SYXK(Hei)2018–007. All rats were kept in a pathogen-free laboratory animal room (temperature: 22 °C ± 2 °C; relative humidity: 53%-62%) with a 12-h light/dark cycle, and they were given standard feed and water ad libitum. The ethical permission was in accordance with standards for the protection of animals used in experiments (Directive 86/609/EEC), and the Animal Ethics Committee of Heilongjiang University of Chinese Medicine reviewed and approved the animal study (Approval No. 2020031203). All procedures were conducted in accordance with the ARRIVE guidelines.
Groups and dosages
After a 10-day acclimatization period, 32 SHR were separated into four groups at random, each with eight rats; According to previous studies that utilized body surface area to convert dosages from humans to animals, the most effective dosage of GJD was selected21, as was the dose of captopril77. Group one (SHR): a hypertension model that was gavaged with 1.5 ml of 0.9% normal saline solution every day at 10 a.m. for 6 weeks. Group two (CAP): gavaged with captopril 13.5 mg/kg/d dissolved in 1.5 ml of 0.9% normal saline solution every day at 10 a.m. for 6 weeks. Group three (GJD): gavaged with GJD powder 5.44 g/kg/d dissolved in 1.5 ml of 0.9% normal saline solution every day at 10 a.m. for 6 weeks. Group four (GJD + CAP): a combination group that gavaged GJD powder 5.44 g/kg/d and captopril 13.5 mg/kg/d dissolved in 1.5 ml of 0.9% normal saline solution every day at 10 a.m. for 6 weeks. The normotensive control (WKY) was given a gavage with an equal volume of 0.9% normal saline solution every day at 10 a.m. for 6 weeks.
Measurement of blood pressure and body weight
Body weight, SBP, and DBP were measured once a week. The blood pressure measuring protocol was created using the approach described in previous studies78,79. The ALC-NIBP, a non-invasive, computed tail-cuff blood pressure measurement and analysis device, was utilized to measure the SBP and DBP. Before the experiment, rats were acclimated to this procedure for one week. A cuff with a pneumatic pulse sensor was affixed to the tail. The ALC-NIBP system automatically measures blood pressure by monitoring the rat's arterial pulse signal with a very sensitive pulse sensor, blocking the arterial pulse with an inflated balloon, and detecting and analyzing changes in the pressure of the balloon and the pulse signal. The blood pressure value selection is based on the cuff pressure when blood flow is blocked, SBP is the pressure at the point when the pulse wave disappears, then automatically decompresses and DBP is the pressure at which the pulse wave begins to weaken. BP was measured first, followed by gavaging; three measures were conducted for each rat, and an average value was recorded. At the end of the experimental period, the rats were weighed accurately and anesthetized using sodium pentobarbital (50 mg/kg, i.p.). The serum, thoracic aorta, kidney, and liver tissue were collected for further analysis.
Biochemical measurement
GJD's safety evolution was examined six weeks after treatment. Creatinine (CRE), blood urea nitrogen (BUN), total protein (TP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), direct bilirubin (DBIL), total bile acid (TBA), and γ-glutamyl transferase (GGT) were all measured in the rats' serum. The kits for these parameters were provided by the Rayto Life and Analytical Sciences Co., Ltd., Shenzhen, and Jiancheng Bioengineering Institute in China, and all procedures were carried out in accordance with the instruction of reagent kits.
ELISA Kits of vasoactive and oxidative stress parameters
All kits (as presented in Table 2) were obtained from the Jiancheng Bioengineering Institute, Nanjing, China, and were measured precisely according to the manufacturer's instructions.
For the determination of AngII and ET-1 levels, the same methods were used by adding samples to the enzyme-labeled wells pre-coated with antibodies, and then biotin-labeled recognition antigens were added and incubated at 37 °C for 30 min. The immune complex was formed and unconjugated biotin antigens were removed by PBST washing. Then, avidin horseradish peroxidase was added and incubated at 37 °C for 30 min. After washing, the combined horseradish peroxidase catalyzed tetramethylbenzidine to blue, which was then converted to yellow by adding the stopped solution of acid. The concentration of AngII and ET-1 was determined by measuring the absorbance at 450 nm. The SOD level was observed by the WST-1 method. SOD catalyzes the disproportionation reaction of the superoxide anion and inhibits the reaction of the superoxide anion produced by xanthine oxidase with WST-1 to produce methazine dye. The activity of SOD was calculated by measuring the absorbance of the reaction product at 450 nm. The MDA level was determined by the TBA method. A red product was obtained when MDA was reacted with thiobarbituric acid (TBA), exhibiting an absorbance peak at 532 nm.
The relatively stable yellow color results from the reaction of GSH with dithiodinitrobenzoic acid to create the 5-thiodinitrobenzoic acid anion was determined by measuring GSH-Px absorbance at 412 nm. The reaction of CAT to break down H2O2 was halted by adding ammonium molybdate to measure the CAT level, and the residual H2O2 interacted with ammonium molybdate to create a light-yellow complex. The absorbance was measured at 405 nm, and then the activity of CAT was calculated per milliliter of serum decomposed 1 μmol of H2O2 per second into an activity unit (U).
qRT-PCR of AGTR1, eNOS, and ET-1
Total RNA was isolated from kidney and thoracic aorta tissues using the Trizol reagent (Takara). For cDNA synthesis, a PrimeScript RT Kit (Takara) was employed. We used TB Green® Premix Ex TaqTM II (Tli RNaseH Plus) in a real-time polymerase chain reaction assay (Takara). The QuantStudioTM3 Real-time PCR technology was used to quantify gene expression levels. The 2-ΔΔCT approach was utilized to analyze and evaluate the data, with GAPDH as a reference gene to calculate and interpret the results. The primer sequences utilized in qRT-PCR are listed in Table 3.
HE staining and Immunohistochemistry
The aorta, kidney, and liver tissues were removed fresh and then fixated with 4% paraformaldehyde at 4° C for 24 h before dehydrating. Fixed tissue was dried, embedded in paraffin, sectioned to a thickness of 5 microns, and stained with hematoxylin–eosin (H&E). A Nikon Eclipse Ci-L (Japan) optical microscope was used to pick the target location of the tissue and to fill the entire visual field with tissue. Each slice had at least three 200× visual fields taken at random and inspected under a microscope. Two veterinary pathologists evaluated HE-stained slides to identify abnormalities. For immunohistochemistry, tissue sections of the thoracic aorta and kidney were deparaffinated in xylene and dehydrated with a series of alcohol. The slices were treated in 3% H2O2 at room temperature for 25 min in the dark to inhibit endogenous peroxidase activity, then rinsed with phosphate-buffered saline (PBS). After 30 min of blocking at room temperature, sections were treated with diluted primary antibodies at 4° C for one night. (AGTR1, ET-1, and eNOS). The slices were then washed in phosphate-buffered saline and incubated for 50 min with the appropriate secondary antibodies. After that, the slices were stained with 3,3-diaminobenzidine and counterstained with hematoxylin (DAB, Servicebio, China). The sections were dehydrated by immersing them in a sequence of solvents until they became transparent and dehydrated. The parts of sections were then mounted with SweSuper Clean BioMount Medium. The primary antibodies utilized for detecting were against the AGTR1 (GB112004, 1:500, Servicebio, China), ET-1 (bs-0954R, 1:500, Bioss, China), and eNOS (GB12086, 1:500, Servicebio, China). The target region of the tissue was selected at random using the light microscope Eclipse Ci-L with a magnification of 200x. Using the pixel area as the reference, values for integrated optical density (IOD) taken from each of the three different slices of the visual field were calculated using the image analysis program Image Pro Plus 6.0.
Statistical analysis
All data is displayed as mean ± standard deviation (Mean ± SD), and it was statistically evaluated using the GraphPad Prism 7.0 software. One-way ANOVA with Tukey's multiple comparisons was used to analyze statistical differences. P values less than 0.05 were considered as statistically significant.
Data availability
The data used to support the findings of this study are included within the supplementary material.
Abbreviations
- GJD:
-
Gedan Jiangya Decoction
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- CVD:
-
Cardiovascular disease
- SHR:
-
Spontaneously hypertensive rats
- WKY:
-
Wistar Kyoto rats
- NO:
-
Nitric oxide
- SOD:
-
Superoxide dismutases
- GSH-Px:
-
Glutathione peroxidase
- CAT:
-
Catalase
- MDA:
-
Malondialdehyde
- ET-1:
-
Endothelin-1
- Ang II:
-
Angiotensin II
- AGTR1:
-
Ang II receptor type 1
- eNOS:
-
Endothelial nitric oxide synthase
- CRE:
-
Creatinine
- BUN:
-
Blood urea nitrogen
- TP:
-
Total protein
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
- DBIL:
-
Direct bilirubin
- TBA:
-
Total bile acid
- GGT:
-
γ-Glutamyl transferase
- ROS:
-
Reactive oxygen species
References
Mills, K. T., Stefanescu, A. & He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 16, 223–237. https://doi.org/10.1038/s41581-019-0244-2 (2020).
Zhou, B., Perel, P., Mensah, G. A. & Ezzati, M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat. Rev. Cardiol. 18, 785–802. https://doi.org/10.1038/s41569-021-00559-8 (2021).
Roth, G. A. et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1736–1788 (2018).
Geldsetzer, P. et al. The state of hypertension care in 44 low-income and middle-income countries: A cross-sectional study of nationally representative individual-level data from 1·1 million adults. Lancet (London, England) 394, 652–662. https://doi.org/10.1016/s0140-6736(19)30955-9 (2019).
Zhou, B. et al. Long-term and recent trends in hypertension awareness, treatment, and control in 12 high-income countries: An analysis of 123 nationally representative surveys. Lancet (London, England) 394, 639–651. https://doi.org/10.1016/s0140-6736(19)31145-6 (2019).
Schutte, A. E., Srinivasapura Venkateshmurthy, N., Mohan, S. & Prabhakaran, D. Hypertension in low- and middle-income countries. Circ. Res. 128, 808–826. https://doi.org/10.1161/circresaha.120.318729 (2021).
Abegaz, T. M., Tefera, Y. G. & Befekadu Abebe, T. Target organ damage and the long term effect of nonadherence to clinical practice guidelines in patients with hypertension: A retrospective cohort study. Int. J. Hypertens. 2017, 2637051. https://doi.org/10.1155/2017/2637051 (2017).
Ramli, A., Ahmad, N. S. & Paraidathathu, T. Medication adherence among hypertensive patients of primary health clinics in Malaysia. Patient Prefer. Adherence 6, 613–622. https://doi.org/10.2147/ppa.S34704 (2012).
Rodrigo, R., González, J. & Paoletto, F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens. Res. 34, 431–440. https://doi.org/10.1038/hr.2010.264 (2011).
Zhang, B. et al. Effect of Chinese medicine xinmaitong on blood pressure in spontaneously hypertensive rats. Cardiol. Res. Pract. 2020, 7869403. https://doi.org/10.1155/2020/7869403 (2020).
Kostov, K. The causal relationship between endothelin-1 and hypertension: Focusing on endothelial dysfunction, arterial stiffness, vascular remodeling, and blood pressure regulation. Life (Basel, Switzerland) https://doi.org/10.3390/life11090986 (2021).
Lin, F. et al. Semen Brassicae reduces thoracic aortic remodeling, inflammation, and oxidative damage in spontaneously hypertensive rats. Biomed. Pharmacother. = Biomedecine & pharmacotherapie 129, 110400. https://doi.org/10.1016/j.biopha.2020.110400 (2020).
Shoorei, H. et al. Evaluation of carvacrol on pituitary and sexual hormones and their receptors in the testicle of male diabetic rats. Hum. Exp. Toxicol. 39, 1019–1030. https://doi.org/10.1177/0960327120909525 (2020).
Basile J., B., M.J. Overview of hypertension in adults, <http://www.uptodate.com> (2022).
Silva, I. V. G., de Figueiredo, R. C. & Rios, D. R. A. Effect of different classes of antihypertensive drugs on endothelial function and inflammation. Int. J. Mol. Sci. https://doi.org/10.3390/ijms20143458 (2019).
Gan, Z. et al. Captopril alleviates hypertension-induced renal damage, inflammation, and NF-κB activation. Braz. J. Med. Biol. Res. = Revista brasileira de pesquisas medicas e biologicas 51, e7338. https://doi.org/10.1590/1414-431x20187338 (2018).
Obied, A. H. H. & Ahmed, A. A. E. Evaluation of the clinical outcome of captopril use for hypertensive urgency in Khartoum State’s emergency centres. Afr. J. Emergency Med. Revue africaine de la medecine d’urgence 11, 202–206. https://doi.org/10.1016/j.afjem.2020.10.003 (2021).
Lai, X. et al. Efficacy and safety of Chinese herbal medicine compared with losartan for mild essential hypertension: A randomized, multicenter, double-blind, noninferiority trial. Circ. Cardiovasc. Quality Outcomes 15, e007923. https://doi.org/10.1161/circoutcomes.121.007923 (2022).
Li, J. Traditional Chinese medicine in treating hypertension. Circul. Cardiovasc. Quality Outcomes 15, e008723. https://doi.org/10.1161/circoutcomes.121.008723 (2022).
Liu, H. et al. Network pharmacology and molecular docking-based mechanism study to reveal antihypertensive effect of Gedan Jiangya Decoction. Biomed. Res. Int. 2022, 3353464. https://doi.org/10.1155/2022/3353464 (2022).
Mohammed, S. A. D. et al. GJD modulates cardiac/vascular inflammation and decreases blood pressure in hypertensive rats. Mediators Inflamm. 2022, 7345116. https://doi.org/10.1155/2022/7345116 (2022).
Shaito, A. et al. Herbal medicine for cardiovascular diseases: Efficacy, mechanisms, and safety. Front. Pharmacol. 11, 422. https://doi.org/10.3389/fphar.2020.00422 (2020).
Mohammed, S. A. D. et al. Integrated Chinese herbal medicine with Western Medicine versus Western Medicine in the effectiveness of primary hypertension treatment: A systematic review and meta-analysis of randomized controlled trials. J. Ethnopharmacol. 300, 115703. https://doi.org/10.1016/j.jep.2022.115703 (2022).
Guerrero-García, C. & Rubio-Guerra, A. F. Combination therapy in the treatment of hypertension. Drugs Context 7, 212531. https://doi.org/10.7573/dic.212531 (2018).
Wald, D. S., Law, M., Morris, J. K., Bestwick, J. P. & Wald, N. J. Combination therapy versus monotherapy in reducing blood pressure: Meta-analysis on 11,000 participants from 42 trials. Am. J. Med. 122, 290–300. https://doi.org/10.1016/j.amjmed.2008.09.038 (2009).
Thomopoulos, C., Parati, G. & Zanchetti, A. Effects of blood-pressure-lowering treatment in hypertension: 9. Discontinuations for adverse events attributed to different classes of antihypertensive drugs: Meta-analyses of randomized trials. J. Hypertens. 34, 1921–1932. https://doi.org/10.1097/hjh.0000000000001052 (2016).
Redon, J., Mourad, J. J., Schmieder, R. E., Volpe, M. & Weiss, T. W. Why in 2016 are patients with hypertension not 100% controlled? A call to action. J. Hypertens. 34, 1480–1488. https://doi.org/10.1097/hjh.0000000000000988 (2016).
Tai, J. et al. Randomized controlled trials of Tianma gouteng decoction combined with Nifedipine in the treatment of primary hypertension: A systematic review and meta-analysis. Evidence Based Complement. Alternative Med. eCAM 2020, 5759083. https://doi.org/10.1155/2020/5759083 (2020).
Mohammed, S. A. D. et al. Integrated Chinese herbal medicine with Western Medicine versus Western Medicine in the effectiveness of primary hypertension treatment: A systematic review and meta-analysis of randomized controlled trials. J. Ethnopharmacol. 300, 115703. https://doi.org/10.1016/j.jep.2022.115703 (2023).
Kim, J. H. et al. Antioxidant effect of captopril and enalapril on reactive oxygen species-induced endothelial dysfunction in the rabbit abdominal aorta. Korean J. Thoracic Cardiovasc. Surg. 46, 14–21. https://doi.org/10.5090/kjtcs.2013.46.1.14 (2013).
Man, S. et al. Antihypertensive and renal protective effect of Shunaoxin pill combined with captopril on spontaneous hypertension rats. Biomedi. Pharmacother. Biomedecine pharmacotherapie 125, 109977. https://doi.org/10.1016/j.biopha.2020.109977 (2020).
Ying, Z. A. & Ping, J. B. Treatment of 78 cases of hyperactive liver-yang hypertension with modified Tianma Gouteng decoction. Shaanxi Tradit Chin. Med. J. 36, 425–427. https://doi.org/10.3969/j.issn.1000-7369.2015.04.018 (2015).
Dasgupta, C. & Zhang, L. Angiotensin II receptors and drug discovery in cardiovascular disease. Drug Discovery Today 16, 22–34. https://doi.org/10.1016/j.drudis.2010.11.016 (2011).
Williamson, C. R., Khurana, S., Nguyen, P., Byrne, C. J. & Tai, T. C. Comparative analysis of renin-angiotensin system (RAS)-related gene expression between hypertensive and normotensive rats. Med. Sci. Monit. Basic Res. 23, 20–24. https://doi.org/10.12659/msmbr.901964 (2017).
Tong, R. C. et al. Extract of Plantago asiatica L. seeds ameliorates hypertension in spontaneously hypertensive rats by inhibition of angiotensin converting enzyme. Front. Pharmacol. 10, 403. https://doi.org/10.3389/fphar.2019.00403 (2019).
Sun, H. J., Wu, Z. Y., Nie, X. W. & Bian, J. S. Role of endothelial dysfunction in cardiovascular diseases: The link between inflammation and hydrogen sulfide. Front. Pharmacol. 10, 1568. https://doi.org/10.3389/fphar.2019.01568 (2019).
Böhm, F. & Pernow, J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc. Res. 76, 8–18. https://doi.org/10.1016/j.cardiores.2007.06.004 (2007).
Dolatkhah, M. A. et al. Fumaria parviflora regulates oxidative stress and apoptosis gene expression in the rat model of varicocele induction. Andrologia 52, e13826. https://doi.org/10.1111/and.13826 (2020).
Griendling, K. K. et al. Oxidative stress and hypertension. Circ. Res. 128, 993–1020. https://doi.org/10.1161/circresaha.121.318063 (2021).
González, J., Valls, N., Brito, R. & Rodrigo, R. Essential hypertension and oxidative stress: New insights. World J. Cardiol. 6, 353–366. https://doi.org/10.4330/wjc.v6.i6.353 (2014).
Briones, A. M. & Touyz, R. M. Oxidative stress and hypertension: Current concepts. Curr. Hypertens. Rep. 12, 135–142. https://doi.org/10.1007/s11906-010-0100-z (2010).
Paravicini, T. M. & Touyz, R. M. Redox signaling in hypertension. Cardiovasc. Res. 71, 247–258. https://doi.org/10.1016/j.cardiores.2006.05.001 (2006).
Rodrigo, R., Passalacqua, W., Araya, J., Orellana, M. & Rivera, G. Implications of oxidative stress and homocysteine in the pathophysiology of essential hypertension. J. Cardiovasc. Pharmacol. 42, 453–461. https://doi.org/10.1097/00005344-200310000-00001 (2003).
Bengtsson, S. H., Gulluyan, L. M., Dusting, G. J. & Drummond, G. R. Novel isoforms of NADPH oxidase in vascular physiology and pathophysiology. Clin. Exp. Pharmacol. Physiol. 30, 849–854. https://doi.org/10.1046/j.1440-1681.2003.03929.x (2003).
Pinheiro, L. C. & Oliveira-Paula, G. H. Sources and effects of oxidative stress in hypertension. Curr. Hypertens. Rev. 16, 166–180. https://doi.org/10.2174/1573402115666190531071924 (2020).
Tsiropoulou, S., Dulak-Lis, M., Montezano, A. C. & Touyz, R. M. in Hypertension and Cardiovascular Disease (ed Emmanuel A. Andreadis) 151–170 (Springer International Publishing, 2016).
Taleb, A. et al. Antioxidant effects and mechanism of silymarin in oxidative stress induced cardiovascular diseases. Biomed. & Pharmacother. = Biomedecine & Pharmacotherapie 102, 689–698. https://doi.org/10.1016/j.biopha.2018.03.140 (2018).
Young, I. S. & Woodside, J. V. Antioxidants in health and disease. J. Clin. Pathol. 54, 176–186. https://doi.org/10.1136/jcp.54.3.176 (2001).
Matés, J. M., Pérez-Gómez, C. & Núñez de Castro, I. Antioxidant enzymes and human diseases. Clin. Biochem. 32, 595–603. https://doi.org/10.1016/s0009-9120(99)00075-2 (1999).
Cao, P. et al. Combination of exercise training and SOD mimetic tempol enhances upregulation of nitric oxide synthase in the kidney of spontaneously hypertensive rats. Int. J. Hypertens. 2020, 2142740. https://doi.org/10.1155/2020/2142740 (2020).
Vaziri, N. D., Lin, C. Y., Farmand, F. & Sindhu, R. K. Superoxide dismutase, catalase, glutathione peroxidase and NADPH oxidase in lead-induced hypertension. Kidney Int. 63, 186–194. https://doi.org/10.1046/j.1523-1755.2003.00711.x (2003).
Alvarez, M. C. et al. Is cardiac hypertrophy in spontaneously hypertensive rats the cause or the consequence of oxidative stress?. Hypertens. Res. 31, 1465–1476. https://doi.org/10.1291/hypres.31.1465 (2008).
Csonka, C. et al. Effects of oxidative stress on the expression of antioxidative defense enzymes in spontaneously hypertensive rat hearts. Free Radical Biol. Med. 29, 612–619. https://doi.org/10.1016/s0891-5849(00)00365-8 (2000).
Armas-Padilla, M. C. et al. Nitric oxide and malondialdehyde in human hypertension. Am. J .Ther. 14, 172–176. https://doi.org/10.1097/01.pap.0000249914.75895.48 (2007).
Yuan, Y. V., Kitts, D. D. & Godin, D. V. Heart and red blood cell antioxidant status and plasma lipid levels in the spontaneously hypertensive and normotensive Wistar-Kyoto rat. Can. J. Physiol. Pharmacol. 74, 290–297 (1996).
Shi, G. et al. Effect of cryptotanshinone on measures of rat cardiomyocyte oxidative stress and gene activation associated with apoptosis. Cardiorenal Med. 11, 18–26. https://doi.org/10.1159/000507184 (2021).
Beltrán-Barrientos, L. M. et al. Mechanistic pathways underlying the antihypertensive effect of fermented milk with Lactococcus lactis NRRL B-50571 in spontaneously hypertensive rats. Nutrients 10, 262. https://doi.org/10.3390/nu10030262 (2018).
Moncada, S., Palmer, R. M. & Higgs, E. A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 43, 109–142 (1991).
Förstermann, U. & Münzel, T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation 113, 1708–1714. https://doi.org/10.1161/circulationaha.105.602532 (2006).
Pinheiro, L. C., Tanus-Santos, J. E. & Castro, M. M. The potential of stimulating nitric oxide formation in the treatment of hypertension. Expert Opin. Ther. Targets 21, 543–556. https://doi.org/10.1080/14728222.2017.1310840 (2017).
Taddei, S., Virdis, A., Ghiadoni, L., Sudano, I. & Salvetti, A. Endothelial dysfunction in hypertension. J. Cardiovasc. Pharmacol. 38(Suppl 2), S11-14. https://doi.org/10.1097/00005344-200111002-00004 (2001).
Kumar, K. V. & Das, U. N. Are free radicals involved in the pathobiology of human essential hypertension?. Free Radical Res. Commun. 19, 59–66. https://doi.org/10.3109/10715769309056499 (1993).
Kumar, C. A. & Das, U. N. Lipid peroxides, anti-oxidants and nitric oxide in patients with pre-eclampsia and essential hypertension. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 6, 901–907 (2000).
Baylis, C., Harvey, J. & Engels, K. Acute nitric oxide blockade amplifies the renal vasoconstrictor actions of angiotension II. J. Am. Soc. Nephrol. 5, 211–214. https://doi.org/10.1681/asn.V52211 (1994).
Wilcox, C. S. Oxidative stress and nitric oxide deficiency in the kidney: A critical link to hypertension?. Am. J. Physiol. Regulatory Integr. Comp. Physiol. 289, R913-935. https://doi.org/10.1152/ajpregu.00250.2005 (2005).
Gómez-Amores, L. et al. L-carnitine attenuates oxidative stress in hypertensive rats. J. Nutr. Biochem. 18, 533–540. https://doi.org/10.1016/j.jnutbio.2006.10.004 (2007).
Maron, B. A. & Michel, T. Subcellular localization of oxidants and redox modulation of endothelial nitric oxide synthase. Circ. J. 76, 2497–2512. https://doi.org/10.1253/circj.cj-12-1207 (2012).
Dzau, V. J., Gibbons, G. H., Morishita, R. & Pratt, R. E. New perspectives in hypertension research. Potentials of vascular biology. Hypertension (Dallas, Tex.:1979) 23, 1132–1140. https://doi.org/10.1161/01.hyp.23.6.1132 (1994).
Giannini, E. G., Testa, R. & Savarino, V. Liver enzyme alteration: A guide for clinicians. CMAJ Can. Med. Assoc. J. = journal de l’Association medicale canadienne 172, 367–379. https://doi.org/10.1503/cmaj.1040752 (2005).
Perrone, R. D., Madias, N. E. & Levey, A. S. Serum creatinine as an index of renal function: New insights into old concepts. Clin. Chem. 38, 1933–1953 (1992).
Iorember, F. M. Malnutrition in chronic kidney disease. Front. Pediatr. 6, 161. https://doi.org/10.3389/fped.2018.00161 (2018).
Ahmadian, S. et al. Toxic effects of VCD on kidneys and liver tissues: A histopathological and biochemical study. BMC. Res. Notes 12, 446. https://doi.org/10.1186/s13104-019-4490-y (2019).
Komatsu, K. et al. Glomerular dynamics and morphology of aged spontaneously hypertensive rats. Effects of angiotensin-converting enzyme inhibition. Hypertension (Dallas, Tex.:1979) 1979(25), 207–213. https://doi.org/10.1161/01.hyp.25.2.207 (1995).
Bolterman, R. J., Manriquez, M. C., Ortiz Ruiz, M. C., Juncos, L. A. & Romero, J. C. Effects of captopril on the renin angiotensin system, oxidative stress, and endothelin in normal and hypertensive rats. Hypertension (Dallas, Tex.:1979) 46, 943–947. https://doi.org/10.1161/01.HYP.0000174602.59935.d5 (2005).
de Cavanagh, E. M., Fraga, C. G., Ferder, L. & Inserra, F. Enalapril and captopril enhance antioxidant defenses in mouse tissues. Am. J. Physiol. 272, R514-518. https://doi.org/10.1152/ajpregu.1997.272.2.R514 (1997).
Chopra, M. et al. Antioxidant effects of angiotensin-converting enzyme (ACE) inhibitors: Free radical and oxidant scavenging are sulfhydryl dependent, but lipid peroxidation is inhibited by both sulfhydryl- and nonsulfhydryl-containing ACE inhibitors. J. Cardiovasc. Pharmacol. 19, 330–340. https://doi.org/10.1097/00005344-199203000-00005 (1992).
Chen, K. et al. Modulation of transforming growth factor-beta signaling pathway mediates the effects of Kangxian Formula on cardiac remodeling. J. Ethnopharmacol. 272, 113922. https://doi.org/10.1016/j.jep.2021.113922 (2021).
Whitesall, S. E., Hoff, J. B., Vollmer, A. P. & D’Alecy, L. G. Comparison of simultaneous measurement of mouse systolic arterial blood pressure by radiotelemetry and tail-cuff methods. Am. J. Physiol. Heart Circ. Physiol. 286, H2408-2415. https://doi.org/10.1152/ajpheart.01089.2003 (2004).
Kubota, Y. et al. Evaluation of blood pressure measured by tail-cuff methods (without heating) in spontaneously hypertensive rats. Biol. Pharm. Bull. 29, 1756–1758. https://doi.org/10.1248/bpb.29.1756 (2006).
Author information
Authors and Affiliations
Contributions
S.M., H.L., and S.L. drafted and designed the manuscript. S.B., P.C., Y.W., and F.L. edited and revised the manuscript. S.M. and H.L. analyzed the data and prepared figures. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohammed, S.A.D., Liu, H., Baldi, S. et al. Antihypertensive, antioxidant, and renal protective impact of integrated GJD with captopril in spontaneously hypertensive rats. Sci Rep 13, 10944 (2023). https://doi.org/10.1038/s41598-023-38020-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-38020-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.