Abstract
Recent studies indicate an association between serum phosphate levels and blood pressure in hypertensive patients. A growing body of evidence suggests that white-coat hypertension (WCH) is associated with target organ damage. Furthermore, metabolic syndrome (MS) and a non-dipping pattern are associated with increased cardiovascular risk. The purpose of this study was to explore the nocturnal blood pressure fall in patients with WCH according to their serum phosphate levels and number of MS components fulfilled. The study included 2600 patients with WCH who attended our outpatient clinics. All patients underwent repeated office blood pressure measurements, 24-h ambulatory blood pressure monitoring and full clinical and laboratory evaluation. The diagnosis of MS was made according to the Adult Treatment Panel III criteria. Dipping pattern was defined as follows: ‘dippers’ had a nocturnal systolic blood pressure (NSBP) fall ⩾10% but <20%; ‘non-dippers’ had an NSBP fall <10%; ‘extreme dippers’ had an NSBP fall ⩾20% and ‘reverse dippers’ had an NSBP increase. There were 314 extreme dippers, 1337 dippers, 734 non-dippers and 116 reverse dippers. Reverse dippers presented with significantly lower levels of serum phosphate, whereas extreme dippers had significantly higher levels (3.39±3.29 vs. 3.58±3.52 mg per 100 ml, P<0.0001). The patients were classified according to the number of MS components and the main observation was the inverse relationship of serum phosphate with MS components (3.53±0.36, 3.50±0.38, 3.49±0.38, 3.44±0.36 and 3.35±0.31 mg per 100 ml, respectively, P=0.003). Patients with WCH and low serum phosphate levels appear to have a higher incidence of a non-dipping NSBP profile and an impaired metabolic profile. This observation may be important for the stratification of the cardiovascular risk in WCH patients.
Similar content being viewed by others
Introduction
Isolated office hypertension or white-coat hypertension (WCH) is defined as persistently elevated office blood pressure (BP), whereas daytime BP, 24-h BP and home BP are in the normal range.1 The definition in some studies was based on the average 24-h ambulatory BP monitoring (ABPM),2, 3 on both systolic BP (SBP) and diastolic BP (DBP) values4, 5, 6 or only on DBP values,7, 8 whereas in other studies, the average ABPM during the day was used.4, 6 Many studies indicate that WCH is associated with target organ damage as well as metabolic abnormalities.9 Increased numbers of metabolic syndrome (MS) components are associated with elevated night-time SBP levels,10 left ventricular hypertrophy7, 11, 12, 13, 14 and increased values of intima-media thickness.15, 16
It is known that phosphate balance is essential for various biological activities and biochemical reactions, and the molecular regulation of phosphate homeostasis has enormous clinical and biological importance.17 Disturbed phosphate balance, such as in acute hypophosphatemia, can cause cardiac dysfunction. Also, chronic hypophosphatemia impairs bone mineralization, resulting in rickets and osteomalacia.18 Furthermore, a growing body of evidence points to an association of serum phosphate levels and BP in hypertensive patients.19, 20, 21
Recently, low serum phosphate levels have been related to the development of hypertension and increased number of components of the MS.22 These findings motivated our investigation of the hypothesis that low serum phosphate levels are associated with a non-dipping pattern and an increased number of metabolic components in WCH patients.
Methods
Study population
The study, which was conducted in the Hypertension Unit of the 1st Cardiology Clinic, Hippokration Hospital, Athens, from the year 2000 to 2009, initially included 12 000 patients who were admitted to our clinics for BP monitoring. The study protocol was approved by the ethics committee of Hippokration Hospital.
The inclusion criteria were patients with office SBP values ⩾140 mm Hg and/or DBP ⩾90 mm Hg, on three consecutive visits to our clinic, each visit 1 week apart, plus mean 24-h ABP values <125/80 mm Hg. Patients with acute or chronic inflammatory disease, endocrine disorders, chronic obstructive pulmonary disease, malignancy, renal insufficiency (serum creatinine >1.5 mg per 100 ml), heart failure, recent (<6 months) cerebrovascular event, coronary artery disease, history of angina, past myocardial infarction, ventricular arrhythmia, sinus bradycardia (<55 b.p.m.), sinus tachycardia (>100 b.p.m.), atrioventicular conduction defects, known diabetes mellitus (earlier antidiabetic treatment) and any condition preventing technically adequate ABPM were excluded from the study. All patients included in the study underwent the following: physical examination, medical history check, repeated clinical BP measurement, blood sampling for routine laboratory examinations and 24-h ABPM. The final cohort included 2600 WCH patients.
BP measurements
The evaluation of BP was made according to the 2007 European Society of Hypertension guidelines published by the European Society of Cardiology for the management of arterial hypertension in individuals aged 18 years or more.1 BP was measured three times with 1 min intervals after a 10–15 min relaxation period with the patient sitting comfortably. A mercury sphygmomanometer was used for all measurements with a medium- or a large-sized cuff, according to the patient's arm circumference.
All patients underwent ABPM for 24 h on the nondominant arm using a Spacelabs 90207 device (Spacelabs, Redmond, WA, USA). The recorder was calibrated with a mercury column and was set to take readings at 20 min intervals from 0600 to 2200 hours, and every 30 min from 2200 to 0600 hours. All patients were encouraged to carry out their normal daily routine after they left the hospital. Daytime and night-time readings corresponded to awake and sleep periods, respectively. The recordings were analyzed to obtain 24-h daytime and night-time average SBP, DBP and heart rates.
According to the 2007 European Society of Hypertension guidelines,1 an average 24-h ABP of 124/79 mm Hg was considered as the upper limit of normality. Each participant was classified according to nocturnal SBP (NSBP) fall as a ‘dipper’ if the decrease was ⩾10% but <20%; a ‘non-dipper’ if it was ⩾0% but <10%; an ‘extreme dipper’ if it was ⩾20% or a ‘reverse dipper’ if NSBP increased compared with the average daytime values.
Anthropometric and biochemical measurements
In each patient, weight, height and waist-to-hip ratio were measured, and body mass index (BMI) was calculated. Waist circumference to the nearest 0.1 cm was measured at the midpoint between the bottom of the rib cage and above the top of the iliac crest from patients at minimal respiration.
All patients underwent full laboratory evaluation (blood test, lipids, liver and kidney function indices). The blood samples were collected from the antecubital vein between 0800 and 1000 hours after a 12 h fast and alcohol abstinence. Serum phosphate and serum calcium levels were measured in the same laboratory with an autoanalyzer (Aeroset; Abbott Laboratories, Chicago, IL, USA) using the end-point photometric method. The reference values of serum phosphate and serum calcium are 2.50–4.30 mg per 100 ml and 8.0–10.6 mEq l−1 (or 16.0–21.20 mg per 100 ml), respectively.
The biochemical evaluation was carried out in the same laboratory, which followed the criteria of the World Health Organization Lipid Reference Laboratories.
Definition of the components of MS
MS was defined according to the Adult Treatment Panel III report23 as three or more of the following criteria being met: (i) abdominal obesity: waist circumference >102 cm in men and >88 cm in women; (ii) hypertriglyceridemia: >150 mg per 100 ml (1.69 mmol l−1); (iii) low high-density lipoprotein cholesterol: <40 mg per 100 ml (1.04 mmol l−1) in men and <50 mg per 100 ml (1.29 mmol l−1) in women; (iv) high BP: SBP >130 mm Hg and/or DBP >85 mm Hg; and (v) high fasting blood glucose: >110 mg per 100 ml. Because of our study inclusion criterion, SBP >140 mm Hg and/or DBP >90 mm Hg, all of our patients fulfilled at least one of the Adult Treatment Panel III criteria. Patients with hypertension only were classified as group I, whereas other groups were as follows: hypertension and one other MS criterion (group II), hypertension and two other MS criteria (group III), hypertension and three other MS criteria (group IV) or all five MS criteria (group V).
Statistical analysis
Statistical analysis was performed using the SPSS package for windows version 13.0 (SPSS, Chicago, IL, USA). Values were expressed as mean±s.d. or as percentages. Means were compared using the independent samples Student's t-test or after analysis of variance when appropriate. Analysis of categorical data was carried out with the χ2-test. Values without normal distribution were analyzed with the Kruskal–Wallis test.
Pearson's correlation coefficients were calculated to examine the univariate relation of phosphate to continuous variables. Comparisons between quartiles of phosphate were performed separately for the five MS groups after analysis of variance and analysis of covariance. Finally, a multivariate logistic analysis of the four groups’ dipping status and the five groups of MS components was performed using phosphate as the dependent variable and age, BMI, waist-to-hip ratio, total cholesterol, triglycerides, high-density lipoprotein, glucose and eGFR as the independent variables. The limit of statistical significance was set at P<0.05.
Results
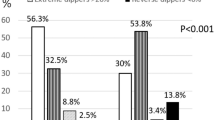
The study cohort was divided into four groups according to NSBP fall: group 1, extreme dippers (n=413); group 2, dippers (n=1337); group 3, non-dippers (n=734) and group 4, reverse dippers (n=116). In the study population, reverse dippers were older and had higher BMI and waist-to-hip values, and significantly lower values of serum phosphate compared to the other three groups. Extreme dippers presented with significantly higher levels of phosphate in comparison to the other three groups. Patients’ baseline characteristics are presented in Table 1. In group 1 (extreme dippers), a strong negative correlation with BMI was noticed (r=−0.1, P=0.043). In group 2 (dippers), there was a negative correlation between waist-to-hip ratio and dipping pattern (r=−0.107, P<0.0001) (Table 2).
Patients were also divided into five groups according to MS components, as described above, and the number of patients in each group was as follows: group I, n=1081; group II, n=858; group III, n=438; group IV, n=191 and group V, n=32. It was observed that as the number of components of MS rose, BMI and waist-to-hip ratio did as well, but the levels of phosphate were significantly higher in the first three groups in comparison to groups IV and V (P=0.003) (Table 3).
In each group, the correlation of phosphate to other parameters was studied. In group I, there was a strong positive correlation of phosphate to calcium and magnesium (r=0.288, P<0.0001; and r=0.107, P<0.001, respectively) and a negative correlation to age, BMI and waist-to-hip ratio (r=−0.063, P=0.039; r=−0.098, P=0.001; and r=−0.09, P=0.003, respectively). In group II, a significant correlation between phosphate and age, calcium and magnesium was observed (r=0.08, P=0.02; r=0.294, P<0.001; and r=0.08, P=0.019, respectively). In group III, a strong positive correlation between phosphate and calcium and BMI was seen (r=0.294, P<0.0001; and r=0.144 P=0.003, respectively), whereas in group V a positive correlation was observed between phosphate and calcium and magnesium (r=0.449, P=0.01; and r=0.627, P<0.001, respectively) (Table 4).
A multivariate analysis was performed in the four groups with dipping status and in the five groups of MS components in which phosphate was set as the dependent variable and age, total cholesterol, high-density lipoprotein, triglycerides, BMI, waist-to-hip ratio and eGFR were the independent variables. Phosphate levels in both models were not significantly affected by these parameters (Table 5).
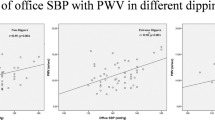
After analysis of covariance, in which phosphate was set as the dependent variable and the model was adjusted for calcium levels, the four groups did not differ in phosphate levels (P=0.712). Finally, calcium and phosphate were multiplied (Ca × P) and divided into quartiles (first <61.38 mg2 dl−2; second 61.38–67.20 mg2 dl−2; third 67.20–72.20 mg2 dl−2; fourth ⩾72.20 mg2 dl−2). Nocturnal BP fall was significantly greater in the highest quartile of Ca × P (P<0.001), whereas there was no correlation among the four dipping pattern groups and Ca × P (P=NS) (Table 6).
Discussion
In this cross-sectional study, we investigated the relationship between different APBM dipping patterns and serum phosphate levels in patients with WCH. It has been reported that low serum phosphate may be related to the development of hypertension and MS.22 We also explored the possible relation of serum phosphate and NSBP dipping status to different distributions of MS components in patients with WCH.
The key finding of this study was that WCH patients with an extreme dipping pattern had higher levels of serum phosphate compared to reverse dippers. Furthermore, when patients were grouped according to the number of the components of the MS fulfilled, individuals with the lowest serum phosphate levels had the highest number of components. In addition, when we used the quartiles of the Ca × P product, the NSBP fall magnitude increased as the levels of the product rose.
The possible mechanisms of the association between low serum phosphate and decreased dipping status in WCH are not yet well established. It is known that WCH may not be entirely benign and that the observed sympathetic hyperactivity may be responsible for the development of target organ damage in these patients.24 Conversely, low serum phosphate has been associated with the development of hypertension explained by the increased sympathoadrenal activity presented in hypertensive patients, because it has been reported that epinephrine leads to a net shift of phosphate from the extracellular to the intracellular compartment.25 In addition, hypophosphatemia in patients with mild essential hypertension appears to be inversely related to sympathetic adrenal tone and may be caused by increased plasma epinephrine within pathophysiologic arterial concentrations.20 Furthermore, serum phosphate is inversely related to BP in normotensive individuals26 when they are compared with age-, sex- and race-matched controls; in our study, serum phosphate levels were lowest in hypertensives.27
The relationship between MS components and low levels of serum phosphate may be causative. It has been previously observed that an unbalanced diet, characterized by low phosphate and high carbohydrate consumption, may lead to reduced serum phosphate levels in patients at risk for the development of MS.28 Increased insulin levels in patients with MS may be a major determinant in this process since insulin stimulates intramyocellular phosphate transport into skeletal muscle, which accounts for much of its hypophosphatemic effects in vivo. Thus reduced phosphate levels may be the consequence of increased tranfer of phosphate from the extracellular to the intacellular compartment.29, 30
The inclusion of Ca × P product in our study was made to add further information to the relation between ion metabolism and WCH. It is known that calcium is essential to neurohumoral control,27 volume regulation31 and vascular smooth muscle function.32 The diverse effects of calcium on BP control reflect its central role in both membrane- and cytosol-associated events. In conjunction with membrane receptors and intracellular calmodulin, calcium regulates cell-to-cell communication, neurotransmitter synthesis and release, and hormone receptor interactions that initiate cytosolic metabolism.33, 34
The role of phosphate in normal cardiovascular physiology is equally as diverse and important as that of calcium and magnesium. In all cells and organs, phosphate is a prerequisite for normal plasma membrane synthesis and integrity.35 In addition, most energy-requiring metabolic functions of a cell are dependent upon phosphate through the formation and degradation of the high energy bonds of ATP. As a consequence, membrane-associated ion pumps, as well as receptor-ion channel interactions, involve phosphate.36 Furthermore, the synthesis, storage and release of local and systemic hormones that regulate cardiac output and vascular resistance require phosphate. In vascular tissue, phosphate is a vital co-factor in the processes outlined above for calcium and magnesium. Thus, the functional role of these ionic species in vascular cell physiology is highly interrelated and the use of Ca × P product may be of more clinical importance.
Study limitations
First, after multivariate analysis, the differences initially observed were not significant. Perhaps the other factors may be stronger in the prediction of dipping status in WCH. Still, this study points to a possible relation between serum phosphate, dipping status and MS components in WCH patients. Second, serum parathyroid hormone levels were not evaluated. Thus, further studies should be performed to elucidate the clinical significance of low serum phosphate values.
In conclusion, patients with WCH and decreased levels of serum phosphate or Ca × P product present with an increased number of MS components and higher night-time SBP levels. This observation may be important because of its prognostic significance in the assessment of the cardiovascular risk of WCH patients. Furthermore, the different phosphate and Ca × P product levels, according to the number of MS components, may reveal various relationships attributed to this syndrome.
References
Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Erdine S, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Lindholm LH, Viigimaa M, Adamopoulos S, Agabiti-Rosei E, Ambrosioni E, Bertomeu V, Clement D, Erdine S, Farsang C, Gaita D, Lip G, Mallion JM, Manolis AJ, Nilsson PM, O’Brien E, Ponikowski P, Redon J, Ruschitzka F, Tamargo J, van Zwieten P, Waeber B, Williams B, Management of Arterial Hypertension of the European Society of Hypertension; European Society of Cardiology. The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). 2007 Guidelines for the management of arterial hypertension. J Hypertens 2007; 25: 1105–1187.
Staessen J, O’Brien E, Atkins N, Amery A . Short report: ambulatory blood pressure in normotensive compared with hypertensive subjects. J Hypertens 1993; 11: 1289–1297.
Cupsidi C, Marabini M, Lonati L, Sampieri L, Comerio G, Pelizzoli S, Leonetti G, Zanchetti A . Cardiac and carotid structure in patients with established hypertension and white-coat hypertension. J Hypertens 1995; 13: 1707–1711.
White WB, Schulman P, McCabe EJ, Dey HM . Average daily blood pressure, not office pressure, determines cardiac function in patients with hypertension. JAMA 1989; 261: 873–877.
Cardillo C, De Felice F, Campia U, Folli G . Psychophysiological reactivity and cardiac end-organ changes in white coat hypertension. Hypertension 1993; 21: 836–844.
Siegel WC, Blumenthal JA, Divine GW . Physiological, psychological and behavioral factors and white coat hypertension. Hypertension 1990; 16: 140–146.
Glen SK, Elliot HL, Curzio JL, Lees KR, Reid JL . White-coat hypertension as a cause of cardiovascular dysfunction. Lancet 1996; 348: 654–657.
Weber MA, Neutel JM, Smith DHG, Graettinger WF . Diagnosis of mild hypertension by ambulatory blood pressure monitoring. Circulation 1994; 90: 2291–2298.
Mancia G, Facchetti R, Bombelli M, Grassi G, Sega R . Long-term risk of mortality associated with selective and combined elevation in office, home and ambulatory blood pressure. Hypertension 2006; 47: 846–853.
Vyssoulis G, Karpanou E, Adamopoulos D, Kyvelou S-M, Gymnopoulou E, Cokkinos D, Stefanadis C . Nocturnal blood pressure fall and metabolic syndrome score in patients with white coat hypertension. Blood Press Monit 2008; 13: 251–256.
Muscholl MW, Hense HW, Brockel U, Doring A, Riegger GAJ, Schunkert H . Changes in left ventricular structure and function in patients with white coat hypertension: cross sectional survey. BMJ 1998; 317: 565–570.
Owens PE, Lyons SP, Rodriguez SA, O’Brien ET . Is elevation of clinic blood pressure in patients with white coat hypertension who have normal ambulatory blood pressure associated with target organ changes? J Hum Hypertens 1998; 12: 743–748.
Palatini P, Penzo M, Canali C, Dorigatti F, Pessina AC . Interactive action of the white-coat effect and the blood pressure levels on cardiovascular complications in hypertension. Am J Med 1997; 103: 208–216.
Palatini P, Mormino P, Santonastaso M, Mos L, Dal Follo M, Zanata G, Pessina AC . Target-organ damage in stage I hypertensive subjects with white coat and sustained hypertension: results from the Harvest Study. Hypertension 1998; 31: 57–63.
O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK . Carotid artery intima and media thickness as a risk factor for myocardial infraction and stroke in older adults. N Engl J Med 1999; 340: 14–22.
Muldoon MF, Nazzaro P, Sutton-Tyrrell K, Manuck SB . White coat hypertension and carotid atherosclerosis. A matching study. Arch Intern Med 2000; 160: 1507–1512.
Berndt T, Thomas LF, Craig TA, Sommer S, Li X, Bergstralh EJ, Kumar R . Evidence for a signaling axis by which intestinal phosphate rapidly modulates renal phosphate reabsorption. Proc Natl Acad Sci USA 2007; 104: 11085–11090.
Sommer S, Berndt T, Craig T, Kumar R . The phosphatonins and the regulation of phosphate transport and vitamin D metabolism. J Steroid Biochem Mol Biol 2007; 103: 497–503.
Kjeldsen SE, Eide I, Os I, Westheim A, Aakesson I, Børre Mogensen S, Frederichsen P, Hjermann I, Gautvik K . Serum phosphate and sympathetic tone in mild essential hypertension. Acta Med Scand Suppl 1986; 714: 119–123.
Kjeldsen SE, Os I, Westheim A, Frederichsen P, Hjermann I, Eide IK, Gautvik K . Decreased serum phosphate in essential hypertension. Related to increased sympathetic tone. Am J Hypertens 1988; 1: 403–409.
Uza G . Hypophosphatemia in patients with essential arterial hypertension. J Trace Elem Electrolytes Health Dis 1990; 4: 245–248.
Gudmundsdottir H, Strand A, Kjeldsen S, Hoieggen A, Os I . Serum phosphate, blood pressure, and the metabolic syndrome-20-year follow-up of middle-aged men. J Clin Hypertens (Greenwich) 2008; 10: 814–821.
Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults. Executive summary. The third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation and treatment of high blood cholesterol in adults (Adult Treatment Panel III). J Am Med Assoc 2001; 285: 2486–2497.
Smith PA, Graham LN, Mackintosh AF, Stoker JB, Mary DA . Sympathetic neural mechanisms in white-coat hypertension. J Am Coll Cardiol 2002; 40: 126–132.
Body JJ, Cryer PE, Offord KP, Heath III H . Epinephrine is a hypophosphatemic hormone in man physiological effects of circulating epinephrine on plasma calcium, magnesium phosphorus, parathyroid hormone and calcitonin. J Clin Invest 1983; 71: 572–578.
Ljunghall S, Hedstrand H . Serum phosphate inversely related to blood pressure. Br Med J 1977; 1: 533.
McCarron DA . Alterations in serum ionized calcium and extracellular binding of calcium in essential hypertension (abstract). Kidney Int 1982; 21: 191.
Haglin L . Hypophosphataemia: cause of the disturbed metabolism in the metabolic syndrome. Med Hypotheses 2001; 56: 657–663.
Riley MS, Schade DS, Eaton RP . Effects of insulin infusion on plasma phosphate in diabetic patients. Metabolism 1979; 28: 191–194.
Bohannon NJ . Large phosphate shifts with treatment for hyperglycemia. Arch Intern Med 1989; 149: 1423–1425.
Blaustein MP, Ratzlaff RW, Kendrick NK . The regulation of intracellular calcium in presynaptic nerve terminals. Ann NY Acad Sci 1978; 307: 195.
Rasmussen H . Cell communication, calcium ion, and cyclic adenosine monophosphate. Science 1970; 170: 404.
Cheung WY . Calmodulin plays a pivotal role in cellular regulation. Science 1979; 207: 19.
Rasmussen H . Calcium and CAMP in stimulus—response coupling. Ann NY Acad Sci 1980; 356: 346.
Lodish HF, Rothman JE . The assembly of cell membranes. Sci Am 1979; 240: 48–63.
DiPolo R . The influence of nucleotidcs on calcium fluxes. Fed Proc 1976; 35: 2579.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vyssoulis, G., Karpanou, E., Tzamou, V. et al. Serum phosphate in white-coat hypertensive patients: focus on dipping status and metabolic syndrome. Hypertens Res 33, 825–830 (2010). https://doi.org/10.1038/hr.2010.86
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2010.86
Keywords
This article is cited by
-
Association of urine phthalate metabolites, bisphenol A levels and serum electrolytes with 24-h blood pressure profile in adolescents
BMC Nephrology (2022)
-
Refeeding and metabolic syndromes: two sides of the same coin
Nutrition & Diabetes (2014)