Abstract
Purpose
To evaluate the choroidal thickness in patients with Graves orbitopathy (GO) using enhanced depth imaging-optical coherence tomography (EDI-OCT).
Methods
Thirty-one patients with GO were evaluated prospectively. All subjects underwent ophthalmologic examination including best-corrected visual acuity, intraocular pressure measurement, biomicroscopic, and fundus examination. Choroidal thickness was measured at the central fovea. In addition, visual evoked potential measurement and visual field evaluation were performed.
Results
The mean choroidal thickness was 377.8±7.4 μ in the GO group, and 334±13.7 μ in the control group. (P=0.004). There was a strong correlation between the choridal thickness and the clinical activity scores (CAS) of the patients (r=0.281, P=0.027). Additionally, there was a correlation between the choroidal thickness and the visual-evoked potential (VEP) P100 latency measurements of the patients (r=0.439, P=0.001).
Conclusions
The results of this study demonstrate that choroid is thicker in patients with GO. The choroidal thickness is also correlated with the CAS and VEP P100 latency measurements in these patients.
Similar content being viewed by others
Introduction
The choroid is the vascular layer of the eye and it provides oxygen and nourishment to the outer layers of the retina. Changes in the choroidal circulation may be related to the pathogenesis of retina, retina pigment epithelium, choroid, and optic nerve. These disorders include hyperopia,1 myopia,2 central serous chorioretinopathy,3 polypoidal choroidal vasculopathy,4 age-related macular degeneration,5 inflammatory eye diseases,6 diabetes,7 glaucoma,8 and scleral buckling surgery.9 The enhanced depth imaging-optical coherence tomography (EDI–OCT) method of spectral domain optical coherence tomography (SD OCT) has enabled the quantitive measurement of choroidal thickness or volume, in addition to obtaining cross-sectional images of choroid.10, 11
Graves orbitopathy (GO), a disorder of autoimmune activity against orbital fibroblasts and adipocytes, is characterized by edema and inflammation of extraocular muscles and an increase in orbital connective tissue and fat. The immune basis of disease is suggested by perivascular and diffuse infiltration of CD4+ and CD8+ T cells, B cells, plasma cells, and macrophages.12 Connective tissues are extensively remodeled with enlargement of the extraocular muscle, muscles, and orbital adipose tissues. The mass effect causes venous obstruction and congestion.
The aim of this study is to investigate whether the changes in the orbital tissues alter the choroidal circulation and subfoveal choroidal thickness in patients with GO. In addition, we aimed to evaluate weather the changes in choroidal thickness correlate with the visual-evoked potential (VEP) measurements and Visual Field examinations of the patients with GO.
Materials and methods
Sixty-two eyes of 31 patients with a diagnosis of GO participated in this prospective study. All patients were euthyroid in both clinical and laboratory examinations. None of the patients were using systemic steroids. The patients were compared with 44 eyes of 22 control subjects who did not have any ophthalmologic or systemic pathology. The study received approval from the Kocaeli University, Faculty of Medicine Ethics Committee. An informed consent was obtained from the patients.
Ophthalmologic examination
All patients and control subjects received comprehensive ocular examination including best-corrected visual acuity, biomicroscopic examination, intraocular pressure (IOP) measurement, indirect ophthalmoscopy, Hertel measurements, and choroidal thickness measurements. The patients with GO additionally underwent visual field tests and VEP measurements.
Clinical activity scores (CAS) of the patients were also evaluated. Patients received one point from each of the symptoms including spontaneous eye pain, eye pain upon eye movement, eyelid swelling, eyelid erythema, conjunctival redness, chemosis, and swollen caruncle. When CAS was 3 points or over, it was defined as active GO.
Exclusion criteria were suggestion of inflammatory orbital disease except GO, previous orbital radiotheraphy, high myopia (greater than −6 diopters), high IOP (>21 mm Hg), and ocular disases that effect choroidal thickness (glaucoma, uveitis, retinal and choroidal diseases).
OCT examination
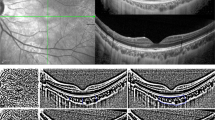
Choroidal thickness was measured using EDI-OCT imaging using spectral domain OCT (Spectralis;Heidelberg Engineering, Heidelberg, Germany). To obtain an EDI-OCT image, the instrument was brought increasingly closer to the eye until we obtained an inverted image. Choroidal thickness, defined as the distance from the inferior margin of the retinal pigment epithelium to the choroid/sclera border was measured at central fovea. After the EDI image is obtained, two blinded clinicians measured the choroidal thickness manually by using calipers. Mean of the two measurements of each patient were taken into consideration. Choroidal thickness was measured at the central fovea (Figure 1).
VEP examination
VEP is defined as variation of bioelectrical potentials in the visual cortex evoked by visual stimuli and evaluates the transmission of neuronal impulses that originate from the retinal photoreceptors passing through visual pathways to the occipital cortex. VEP is obtained by averaging the responses from occipital scalp electrodes generated by 100 or more sequential stimuli. The VEP is generated using a black-white checkered-board pattern on a television monitor. Patients sat one meter away from the monitor. The patients’ gaze was fixed on a point at the center of the television monitor monocularly. The bioelectric signal was filtered (bandpass 0.5–200 Hz). Two hundred responses were averaged for every trial (Neuropack Nihon Kohden, MEB-5504 K, Tokyo, Japan). The analysis time was 250 msec. Sweep length was 300 msec (30 msec/div) and stimulus rate was 1 Hz. Peak latencies of the P100 component were measured.
Statistical analysis
All statistical analyses were performed using software IBM SPSS Statistics 19.0 version (SPSS, Inc., Chicago, IL, USA). The results were reported as mean values±SD. Choroidal measurements of the GO and control eyes were compared by using independent samples t test. The significance of correlations between the choroidal thickness, VEP, mean deviation (MD) of visual field and CAS were determined by Pearson’s correlation coefficient test. A P<0.05 was considered statistically significant.
Results
Mean age of the GO group and controls were 44.13±2.2 and 45.3±3.1, respectively (P=0.747). Twenty-four patients were female and 8 patients were male in the GO group. There was no statistical difference in gender between the groups (P>0.05). Eleven patients demonstrated the CAS evaluated as 0, six patients as 1, five patients as 2, five patients as 3, and four patients as 4. There was no patient with CAS of 5,6 or 7. None of the patients had decrease in visual acuity related with the GO. The IOP of the patients were within the normal limits. The clinical characteristics of the patients were summarized in Table 1.
The mean choroidal thickness was 377.8±7.4 μ in the GO group, and 334±13.7 μ in the control group (P=0.004). We correlated the CAS of the patients with their choroidal thickness. We observed that choroid was thicker in patients with higher CAS. The correlation between these two parameters were statistically significantly different (r=0.281, P=0.027; Figure 2).
We correlated the VEP measurements of the patients with their choroidal thickness. We found that choroid was thicker in patients with elongated VEPp100 measurements (r=0.439, P=0.001; Figure 3).
We also evaluated the correlation between choroidal thickness and MD in visual field examination. The choroid thickness increased as the MD decreased. However, this correlation did not reach to the statistical significance (r=−0.273, P=0.061).
Discussion
OCT is a non-invasive method that is used for quantitive assessment of retinal morphology, and it offers valuable data about the prognosis of the patients. A relatively new technique, EDI-OCT uses light with a longer wavelength, which is more effective for choroidal scanning and provides valuable data about choroidal morphology. Recently, many SD OCT instruments have been used the EDI method for the chorodal thickness measurements in various diseases.3, 13, 14 Changes in the choroidal thickness might be related to the pathogenesis of retinal, retina pigment epithelium, and optic nerve diseases. These studies reported increased choroidal thickness in patients with posterior uveitis including Vogt-Koyanagi Harada disease, multiple evanescent white dot syndrome and Behcet’s disease.15, 16, 17 In this study we found that subfoveal choroidal thickness of eyes with GO was significantly higher than normal eyes.
Patients with GO commonly have evidence of both extra-ocular muscle and orbital adipose tissue involvement. The hallmark of GO is swelling of the extraocular muscles in association with an increase in orbital connective tissue and fat volume to a variable extend. The expanded orbital tissues causes increase in intra-orbital pressure. As a result, the venous outflow from the orbit is impeded.18 Elevated episcleral venous pressure value has been demonstrated in GO, and raised retrobulbar pressure above normal venous pressure has been reported as a possible cause of reduced orbital venous drainage.19, 20 Konuk et al21 found that the superior ophthalmic vein blood flow velocity was significantly lower in severe GO cases compared with modere and mild ones. The increase in venous pressure may lead to an elevation with IOP in some patients with GO.22 In addition, it has been show that orbital decompression reduces the IOP and superior ophthalmic vein velocity significantly.23 None of our patients had severe GO, and the mean IOP was within the normal limits. However, the mean choroidal thickness was elevated. We think that choroidal thickness might be affected from the venous obstruction and congestion in patients with GO. The elevation of the choroidal thickness might be an early sign of venous congestion that occurs before the elevation of IOP.
Another mechanism that might alter the thickness of choroid might be the direct compression of the globe. Chroidal folds might occur with extraocular process that induces sufficient compressive stress within the choroid.24 Jorge et al25 reported that choroidal folds might resolve and visual acuity might increase after orbital decompression for GO despite persistent enlargement of extraocular muscles. They claim that tension on the choriocapillaris could be generated by direct pressure on the sclera by enlarged eye muscles.25 Odrobina et al9 found that subfoveal choroidal thickness of eyes after scleral buckling surgery using an encircling band, in long-term observation, was significantly thicker. They claim that scleral buckling reduces blood flow and increases hemostasis in choroidal circulation. This may cause an elevation in choroidal pressure and may increase the subfoveal choroidal thickness.9
The compression of the optic nerve by an orbital apex crowding often results with the optic neuropathy in patients with GO. This condition occurs in ~5% of the patients with GO.26 It might be asymptomatic with good vision, but it might also manifest with decreased visual acuity, reduced color vision, visual field defects, or afferent pupillary defects. If these symptoms and signs are not present, the patient might be unaware of visual loss until advanced clinical changes have occurred. VEP might help us gaining objective information about the optic nerve dysfunction in GO.27, 28, 29 Weis et al30 evaluated the relationship between the orbital bony geometry and the volume of the intraorbital structures in predicting optic neuropathy in GO. They found that medial rectus muscle size was a predictor of compressive optic neuropathy.30 As compression on the optic nerve is also related with the stasis in the choroid, we hypothesize that increase in extra-ocular volume might also be related with the increase in choroidal thickness. In our study, none of the patients had the clinical signs of optic neuropathy. However, we found that choroid was thicker in patients with longer VEP latency. We believe that choroidal thickness measurements might be used to discriminate the early changes in optic nerve in patients with GO. Further investigations are needed to understand the relationship between these two parameters.
GO is an inflammatory and autoimmune disorder of the orbit. The immune basis of the disease is composed by a perivascular and diffuse infiltration of CD4+ and CD8+ T cells, B cells, plasma cells, and macrophages.31 Orbital fibroblasts are thought to have crucial role in the pathogenesis by their ability to produce hydrophilic glycosaminoglycans that result in retention of fluid and edematous swelling of orbital tissues. The immune activity of ophthalmopathy is neither synonymous nor coincident with clinical severity of the disease.32 Several methods have been proposed to evaluate disease activity; none has yet become generally accepted for routine use in clinical practice. The CAS has been shown to be a useful method, in spite of sensitivity and inter-observer variation weakness. Mourits et al33 found good correlation between initial and post immune-suppressive treatment CAS. In another study, Tortora et al34 evaluated correlation between the orbital MRI imaging and CAS. They found a statistical correlation between CAS and both STIR and contrast enhanced T1-weighted sequences.34 In our study, we found that choroid was thicker in patients with higher CAS. However, our patients’ CAS scores were distributed between 0 and 4. We believe that comparison of choridal thickness of the patients with higher CAS might give a better idea in relationship between CAS and choroidal thickness. Absence of patients with higher CAS might be considered as a weakness in our study.
In conclusion, we found that patients with GO have higher choroidal thickness than the healthy controls, and we observed that choroid was thicker in patients with higher CAS. In addition, VEP measurements of the patients were correlated with the choroidal thickness of the patients. Choroidal thickness measurement might be a beneficial test for evaluating GO, and discriminating optic neuropathy. The patients in our study did not have severe GO and they did not have high CAS. We believe that further investigations with a larger and variable group of patients would show more disease-specific results.

References
Hefner L, Gerding H . Analysis of choroidal folds of the posterior pole in excessive hyperopia using SD-OCT with enhanced depth imaging (EDI). Klin Monbl Augenheilkd 2012; 229 (4): 403–406.
Fujiwara T, Imamura Y, Margolis R, Slakter JS, Spaide RF . Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am J Ophthalmol 2009; 148 (3): 445–450.
Imamura Y, Fujiwara T, Margolis R, Spaide RF . Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina 2009; 29 (10): 1469–1473.
Koizumi H, Yamagishi T, Yamazaki T, Kawasaki R, Kinoshita S . Subfoveal choroidal thickness in typical age-related macular degeneration and polypoidal choroidalvasculopathy. Graefes Arch Clin Exp Ophthalmol 2011; 249 (8): 1123–1128.
Switzer Jr DW, Mendonça LS, Saito M, Zweifel SA, Spaide RF . Segregation of ophthalmoscopic characteristics according to choroidal thickness in patients with early age-related macular degeneration. Retina 2012; 32 (7): 1265–1271.
da Silva FT, Sakata VM, Nakashima A, Hirata CE, Olivalves E, Takahashi WY et al. Enhanced depth imaging optical coherence tomography in long-standing Vogt-Koyanagi-Harada disease. Br J Ophthalmol 2013; 97 (1): 70–74.
Esmaeelpour M, Brunner S, Ansari-Shahrezaei S, Nemetz S, Povazay B, Kajic V et al. Choroidal thinning in diabetes type 1 detected by 3-dimensional 1060 nm optical coherence tomography. Invest Ophthalmol Vis Sci 2012; 53 (11): 6803–6809.
Maul EA, Friedman DS, Chang DS, Boland MV, Ramulu PY, Jampel HD et al. Choroidal thickness measured by spectral domain optical coherence tomography: factors affecting thickness in glaucoma patients. Ophthalmology 2011; 118 (8): 1571–1579.
Odrobina D, Laudańska-Olszewska I, Gozdek P, Maroszyński M, Amon M . Influence of scleral buckling surgery with encircling band on subfoveal choroidal thickness in long-term observations. Biomed Res Int 2013; 2013: 586–894.
Spaide RF, Kouzumi H, Pozzoni MC . Enhanced depth imaging spectral domain optical coherence tomography. Am J Ophthalmol 2008; 146 (4): 496–500.
Branchini L, Regatieri CV, Flores-Moreno I, Baumann B, Fujimoto JG, Duker JS . Reproducibility of choroidal thickness measurements across three spectral domain optical coherence tomography systems. Ophthalmology 2012; 119 (1): 119–123.
McLachlan SM, Prummel MF, Rapaport B . Cell-mediated or humoral immunity in Graves' ophthalmopathy? Profiles of T-cell cytokines amplified by polymerase chain reaction from orbital tissue. J Clin Endocrinol Metab 1994; 78 (5): 1070–1074.
Karampelas M, Sim DA, Keane PA, Zarranz-Ventura J, Patel PJ, Tufail A et al. Choroidal assessment in idiopathic panuveitis using optical coherence tomography. Graefes Arch Clin Exp Ophthalmol 2013; 251 (8): 2029–2036.
Zhou M, Wang W, Huang W, Gao X, Li Z, Li X et al. Is increased choroidal thickness association with primary angle closure? Acta Ophthalmol 2014; 92: e514–e520.
Fong AH, Li KK, Wong D . Choroidal evaluation using enhanced depth imaging spectral domain optical coherence tomography in Vogt-Koyanagi-Harada disease. Retina 2011; 31: 502–509.
Aoyagi R, Hayashi T, Masai A, Mitooka K, Gekka T, Kozaki K et al. Subfoveal choroidal thickness in multiple evanescent white dot syndrome. Clin Exp Optom 2012; 95: 212–217.
Coskun E, Gurler B, Pehlivan Y, Kisacik B, Okumus S, Yayuspayı R et al. Enhanced depth imaging optical coherence tomography findings in Behçet disease. Ocul Immunol Inflamm 2013; 21 (6): 440–445.
Nakase Y, Osanai T, Yoshikawa K, Inoue Y . Color Doppler imaging of orbital venous flow in dysthyroid optic neuropathy. Jpn J Ophthalmol 1994; 38: 80–86.
Otto AJ, Koornneef L, Mourits MP, Deen-van Leuwen L . Retrobulbar pressures measured during surgical decompression of the orbit. Br J Ophthalmol 1996; 80: 1042–1045.
Somer D, Ozkan SB, Ozdemir H, Atilla S, Söylev MF, Duman S . Colour Doppler imaging of superior ophthalmic vein in thyroid associated eye disease. Jpn J Ophthalmol 2002; 46: 341–345.
Konuk O, Onaran Z, Oktar SO, Yucel C, Unal M . Intraocular pressure and superior ophthalmic vein velocity in Graves’ orbitopathy: relation with clinical features. Graefes Arch Clin Exp Ophthalmol 2009; 247: 1555–1559.
Dev S, Damji KF, DeBacker CM, Cox TA, Dutton JJ, Allingham RR . Decrease in intraocular pressure after orbital decompression for thyroid orbitopathy. Can J Ophthalmol 1998; 33 (6): 314–319.
Onaran Z, Konuk O, Oktar SÖ, Yücel C, Unal M . Intraocular pressure lowering effect of orbital decompression is related to increased venous outflow in Graves orbitopathy. Curr Eye Res 2014; 39 (7): 666–672.
Friberg TR . The etiology of choroidal folds. Graefes Arch Clin Exp Ophthalmol 1989; 227: 459–464.
Jorge R, Scott IU, Akaishi PMS, Cruz AAV, Flynn HW . Resolution of choroidal folds and improvement in visual acuity after orbital decompression for Graves Orbitopathy. Retina 2003; 23 (4): 563–565.
Lucarelly MJ, Shore JW . Management of thyroid orbitopathy. Int Ophthalmol Clin 1996; 36: 179–193.
Setela K, Raitta C, Valimaki M, Katevuo V, Lamberg RA . The value of visual evoked potentials in optic neuropathybof Graves’ disease. J Endocrinol Invest 1992; 15: 821–826.
Batch JA, Lepre F . Early diagnosis of Graves’ optic neuropathy using visual evoked responses. Postgrad Med J 1990; 66: 664–666.
Boback P, Friedman R, Mitchell B, Goodwin J, Anderson R . Visual evoked potentials to multiple temporal frequencies. Arch Ophthalmol 1988; 106: 936–940.
Weis E, Heran MK, Jhamb A, Chan AK, Chiu JP, Hurley MC et al. Quantitive computed tomographic predictors of compressive optic neuropathy in patients with thyroid orbitopathy: a volumetric analysis. Ophthalmology 2012; 119: 2174–2178.
McLahlan SM, Prummel MF, Rappaport B . Cell mediated or humoral immunity in Graves’ ophthalmopathy? Profiles of T cell cytokines amplified by polymerase chain reaction from orbital tissue. J Clin Endocrinol Metab 1994; 78: 1070–1074.
Wiersinga W, Prummel MF . Therapeutic controversies. Retrobulbar radiation in Grave’s ophthalmopathy. J Clin Endocrinol Metab 1995; 80: 345–347.
Mourits MP, Prummel MF, Wiersinga WM, Koornneef L . Clinical activity score as a guide in the management of patients with Graves' ophthalmopathy. Clin Endocrinol 1997; 47: 9–14.
Tortora F, Cirillo M, Ferrara M, Belfiore MP, Carella C, Caranci F et al. Disease activity in Graves' ophthalmopathy: diagnosis with orbital MR imaging and correlation with clinical score. Neuroradiol J 2013; 26 (5): 555–564.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Özkan, B., Koçer, Ç., Altintaş, Ö. et al. Choroidal changes observed with enhanced depth imaging optical coherence tomography in patients with mild Graves orbitopathy. Eye 30, 917–924 (2016). https://doi.org/10.1038/eye.2016.93
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2016.93
This article is cited by
-
Correlation between choroidal vascularity and retrobulbar ocular blood flow changes and thyroid-associated ophthalmopathy activity: a cross-sectional study
BMC Ophthalmology (2024)
-
Automated evaluation of parapapillary choroidal microvasculature in thyroid eye disease
International Ophthalmology (2023)
-
Increase of central foveal and temporal choroidal thickness in patients with inactive thyroid eye disease
BMC Ophthalmology (2021)
-
Choroidal vascularity index in thyroid-associated ophthalmopathy: a cross-sectional study
Eye and Vision (2021)
-
Evaluation of retinal and choroidal variations in thyroid-associated ophthalmopathy using optical coherence tomography angiography
BMC Ophthalmology (2020)