The treatment of many melanomas is now starting with immune checkpoint inhibitors, rather than major surgery. Credit: Anne Weston, Francis Crick Institute
When Jedd Wolchok began working in the area of melanoma 20 years ago, the average life expectancy for a patient with advanced disease was six or seven months.
Now his waiting room is full of people coming back for their third or fourth year of follow-up, sharing their stories of survival with the newly diagnosed, giving hope where just a decade ago there was little.
“That gives you a sense of the human impact of this,” says Wolchok, a medical oncologist and director of the Parker Institute for Cancer Immunotherapy at the Memorial Sloan Kettering Cancer Center in New York, ranked fifth in the Nature Index for cancer research output.
Transformative treatment
Behind this transformation in melanoma survival rates is a class of drugs called checkpoint inhibitors, the first of which was approved nine years ago. Checkpoint inhibitors are a form of cancer immunotherapy — treatments that stimulate the immune response to cancer cells. Checkpoint inhibitors are not the first form of cancer immunotherapy, but they are, so far, among the most successful, particularly in melanoma. They’re also having a big impact in lung and urinary tract cancers. “Melanoma is the most sensitive type of cancer to checkpoint inhibitors,” says James Larkin, medical oncologist at the Royal Marsden Hospital in London. But no one is sure why. Some patients respond well to checkpoint inhibitors, but others don’t respond at all, for reasons that are also not yet understood.
Checkpoint inhibitors work by preventing tumour cells from hijacking, and therefore avoiding, the cellular immune response that should eliminate them. Their discovery came about in the late 1990s, when two groups of researchers from the United States and Japan uncovered a series of interactions between cell-surface receptors and proteins that led to the death of immune T cells.
T cells are the cells that would normally lead the charge against cancer and other threats. They have a receptor on their surface called PD-1 (programmed cell death protein 1). When that receptor is engaged, it triggers the T cell to rupture — one of the many checkpoints that have evolved to help keep the immune system from over-reacting.
The protein that engages that receptor is PD-L1 (PD ligand 1). It turns out that many human cancers also produce PD-L1, the factor that tumours are using to hijack the checkpoint and engage the T-cell death receptor to stop the response against them.
Scientists showed that inhibiting this tumour-hijacked checkpoint could unleash an immune response against the tumour.
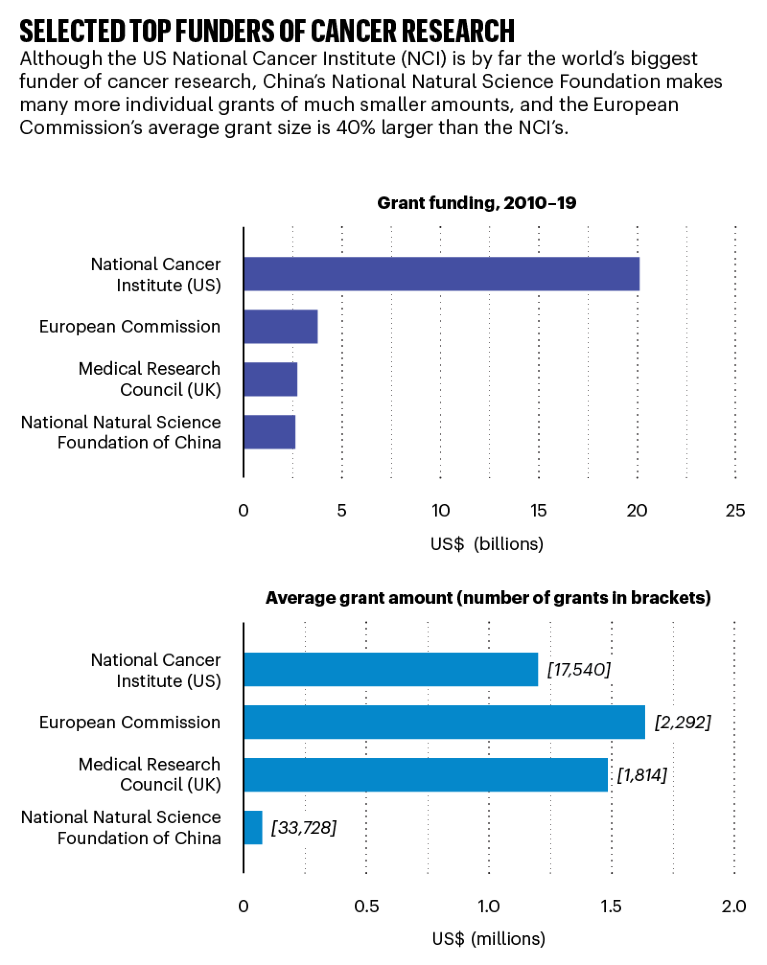
Source: Dimensions, an interlinked research information system provided by Digital Science (https://www.dimensions.ai)
A sense of possibility
The first checkpoint inhibitor drug, ipilimumab, was approved by the US Food and Drug Administration in March 2011 for the treatment of melanoma that had spread or that could not be treated surgically. Compared with a melanoma vaccine, itself a new therapeutic approach being trialled, the drug significantly improved survival rates. Although it worked in only around one in five patients, the benefits in those patients were dramatic, Larkin says. “We really had a sense then of the possibilities.”
Ipilimumab was followed by pembrolizumab in September 2014, and nivolumab just three months later. All of these, and newer checkpoint inhibitors, are now in widespread use, although they’re expensive for patients, particularly in countries without public health insurance schemes. A course of intravenous checkpoint inhibitor therapy can cost US$150,000–250,000 per year.
The most spectacular results so far with checkpoint inhibitor therapy have come from trials combining two different checkpoint inhibitors, such as ipilimumab and nivolumab. Larkin and Wolchok were both involved in the CheckMate 067 study, which began in July 2013 and compared ipilimumab alone with nivolumab alone, and with ipilimumab plus nivolumab in 945 people with advanced untreated melanoma.
“It was a blinded trial, so you didn’t know which treatment the patients were getting,” Larkin says. “And it was really striking that some patients who had symptoms or were quite sick were improving really, really quickly, which we’d never seen before.”
The combination was so successful that a paper published in the New England Journal of Medicine in late 2019 showed that 52% of patients were alive after five years, compared with 44% of patients on nivolumab alone and 26% of patients on ipilimumab alone (J. Larkin et al. N. Engl. J. Med. 381, 1535–1546; 2019). As often with clinical trials, checkpoint inhibitors were first tested in the most severely affected patients, those whose cancer was untreatable with surgery or which had spread despite existing treatments. But with each new trial showing unprecedented survival rates, questions would arise as to whether these drugs should be used earlier in the disease, even before it had spread.
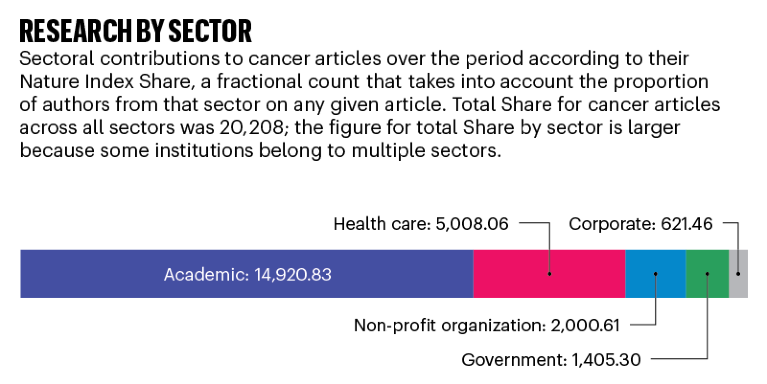
Source: Dimensions, an interlinked research information system provided by Digital Science (https://www.dimensions.ai)
Grant McArthur, a medical oncologist and head of the molecular oncology laboratory at the Peter MacCallum Cancer Centre in Melbourne, Australia, says checkpoint inhibitors have brought a paradigm shift in the management of melanoma. “We see patients, who previously would have had large, complex surgical procedures that are associated with substantial morbidity, who now will start with the immune checkpoint inhibitors,” he says. “The idea that immunotherapy could replace surgery is being entertained for the first time.”
It’s not all good news. Checkpoint inhibitors come with some potentially serious side effects, many as a result of an over-active immune response, which is linked to inflammation in the bowel, lung, heart, skin and other organs. And around half of the patients with advanced disease don’t respond as spectacularly, or at all, to checkpoint inhibitors.
Some survive longer than they might have done without treatment, or have a longer period until their disease progresses. However, the CheckMate 067 study found that 48% of patients had died within five years, despite treatment with a combination of checkpoint inhibitors. There’s palpable frustration over why no one can explain this. It’s an active area of research, and there are early suggestions about what might be the deciding factors. One clue is that people who seem to get the most benefit from checkpoint inhibitors are those whose immune systems are already putting up a fight when they start treatment, says Wolchok.
“The best evidence for that comes from pathology studies, which have shown that tumours that already have T cells in them are the ones where you see responses,” he says. “What the checkpoint inhibitors are doing in general is allowing a pre-existing immune response to become more effective.”
There’s also evidence that patients with cancers caused by a certain genetic condition called mismatch repair deficiency may actually respond better to checkpoint inhibitors, regardless of their cancer type.
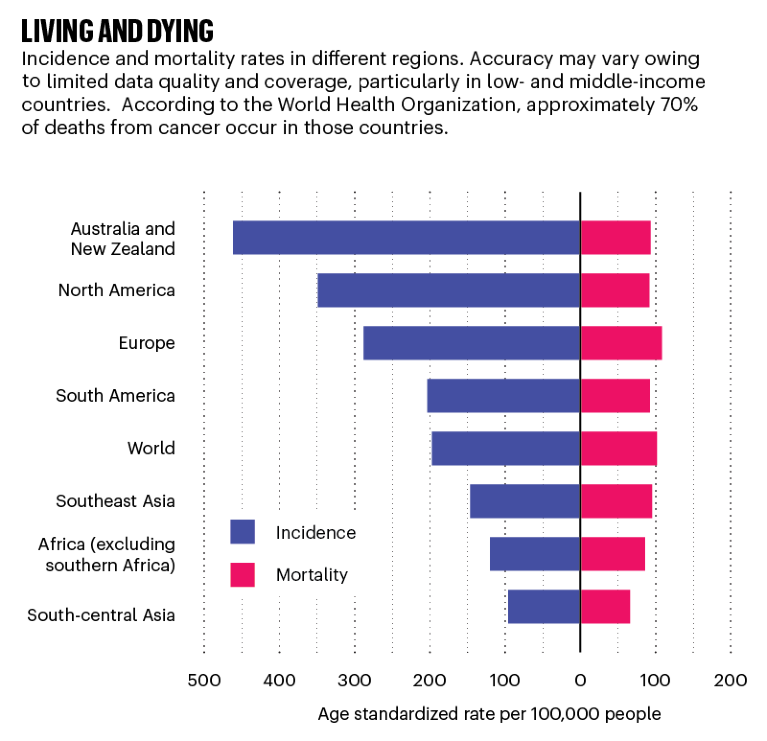
Source: Globoscan 2018/WHO
Into the unknown
Another feature that seems to be linked to better response rates is what’s called the mutation burden of the tumour, the number of genetic mutations present in the genome of an individual’s cancer. Just as exposure to cigarette smoke causes the mutations that are common to lung cancers, exposure to ultraviolet radiation causes a set of mutations that are common features of skin cancer. But individuals with skin cancer that grows in parts of the body that are less exposed to the sun may have a lower mutation burden, and that seems to make them less likely to respond to checkpoint inhibitors.
“The hypothesis is that cancers that have a lot of mutations have many abnormal-appearing proteins, which makes them look different from the normal cell that they came from,” says Wolchok. “That is something that the immune system at baseline is able to survey for.”
Given the survival rates among people who do respond to checkpoint inhibitors, is it time to start talking about a cure for melanoma? Oncologists are wary of the word, preferring to talk about long-term survivorship, which is itself a novel concept in melanoma.
“If you’ve no longer got a disease that 20 years ago had a survival of six to nine months, and it turns out that you’re a long-term survivor, what does that look like?” Larkin asks. “Curing metastatic solid tumours isn’t something that we’ve ever really faced before.”


 A global drive to eliminate cervical cancer
A global drive to eliminate cervical cancer
 Worth the cost? A closer look at the da Vinci robot’s impact on prostate cancer surgery
Worth the cost? A closer look at the da Vinci robot’s impact on prostate cancer surgery
 Three researchers who are coming at cancer from all angles
Three researchers who are coming at cancer from all angles