- NEWS
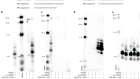
Two Ebola drugs show promise amid ongoing outbreak
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
doi: https://doi.org/10.1038/d41586-019-02442-6

 Science under fire: Ebola researchers fight to test drugs and vaccines in a war zone
Science under fire: Ebola researchers fight to test drugs and vaccines in a war zone
 Ebola outbreak declared an international public-health emergency
Ebola outbreak declared an international public-health emergency
 The doctor who beat Ebola — and inspires other survivors to care for the sick
The doctor who beat Ebola — and inspires other survivors to care for the sick
 Meet the Ebola workers battling a virus in a war zone
Meet the Ebola workers battling a virus in a war zone