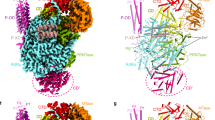
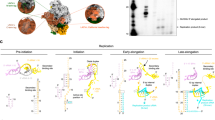
Abstract
Non-segmented negative-strand RNA viruses, including Ebola virus (EBOV), rabies virus, human respiratory syncytial virus and pneumoviruses, can cause respiratory infections, haemorrhagic fever and encephalitis in humans and animals, and are considered a substantial health and economic burden worldwide1. Replication and transcription of the viral genome are executed by the large (L) polymerase, which is a promising target for the development of antiviral drugs. Here, using the L polymerase of EBOV as a representative, we show that de novo replication of L polymerase is controlled by the specific 3′ leader sequence of the EBOV genome in an enzymatic assay, and that formation of at least three base pairs can effectively drive the elongation process of RNA synthesis independent of the specific RNA sequence. We present the high-resolution structures of the EBOV L–VP35–RNA complex and show that the 3′ leader RNA binds in the template entry channel with a distinctive stable bend conformation. Using mutagenesis assays, we confirm that the bend conformation of the RNA is required for the de novo replication activity and reveal the key residues of the L protein that stabilize the RNA conformation. These findings provide a new mechanistic understanding of RNA synthesis for polymerases of non-segmented negative-strand RNA viruses, and reveal important targets for the development of antiviral drugs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The cryo-EM density maps and atomic coordinates have been deposited to the Electron Microscopy Data Bank and the Protein Data Bank, respectively. The accession numbers are as follows: EBOV L–VP35–RNA complex focus on polymerase (8JSL; EMD-36622); EBOV L–VP35–RNA complex in conformation 1 (8JSM; EMD-36623); and EBOV L–VP35–RNA complex in conformation 2 (8JSN; EMD-36624). Source data are provided with this paper.
References
Ouizougun-Oubari, M. & Fearns, R. Structures and mechanisms of nonsegmented, negative-strand RNA virus polymerases. Annu. Rev. Virol. https://doi.org/10.1146/annurev-virology-111821-102603 (2023).
te Velthuis, A. J. W., Grimes, J. M. & Fodor, E. Structural insights into RNA polymerases of negative-sense RNA viruses. Nat. Rev. Microbiol. 19, 303–318 (2021).
Pyle, J. D., Whelan, S. P. J. & Bloyet, L. M. Structure and function of negative-strand RNA virus polymerase complexes. Enzymes 50, 21–78 (2021).
Wandzik, J. M. et al. A structure-based model for the complete transcription cycle of influenza polymerase. Cell 181, 877–893 (2020).
Pflug, A., Guilligay, D., Reich, S. & Cusack, S. Structure of influenza A polymerase bound to the viral RNA promoter. Nature 516, 355–360 (2014).
Reich, S. et al. Structural insight into cap-snatching and RNA synthesis by influenza polymerase. Nature 516, 361–366 (2014).
Hengrung, N. et al. Crystal structure of the RNA-dependent RNA polymerase from influenza C virus. Nature 527, 114–117 (2015).
Peng, Q. et al. Structural insight into RNA synthesis by influenza D polymerase. Nat. Microbiol. 4, 1750–1759 (2019).
Arragain, B. et al. Pre-initiation and elongation structures of full-length La Crosse virus polymerase reveal functionally important conformational changes. Nat. Commun. 11, 3590 (2020).
Arragain, B. et al. Structural snapshots of La Crosse virus polymerase reveal the mechanisms underlying Peribunyaviridae replication and transcription. Nat. Commun. 13, 902 (2022).
Wang, P. et al. Structure of severe fever with thrombocytopenia syndrome virus L protein elucidates the mechanisms of viral transcription initiation. Nat. Microbiol. 5, 864–871 (2020).
Vogel, D. et al. Structural and functional characterization of the severe fever with thrombocytopenia syndrome virus L protein. Nucleic Acids Res. 48, 5749–5765 (2020).
Xu, X. et al. Cryo-EM structures of Lassa and Machupo virus polymerases complexed with cognate regulatory Z proteins identify targets for antivirals. Nat. Microbiol. 6, 921–931 (2021).
Peng, R. C. et al. Structural insight into arenavirus replication machinery. Nature 579, 615–619 (2020).
Kouba, T. et al. Conformational changes in Lassa virus L protein associated with promoter binding and RNA synthesis activity. Nat. Commun. 12, 7018 (2021).
Liang, B. et al. Structure of the L protein of vesicular stomatitis virus from electron cryomicroscopy. Cell 162, 314–327 (2015).
Jenni, S. et al. Structure of the vesicular stomatitis virus L protein in complex with its phosphoprotein cofactor. Cell Rep. 30, 53–60 (2020).
Horwitz, J. A., Jenni, S., Harrison, S. C. & Whelan, S. P. J. Structure of a rabies virus polymerase complex from electron cryo-microscopy. Proc. Natl Acad. Sci. USA 117, 2099–2107 (2020).
Pan, J. H. et al. Structure of the human metapneumovirus polymerase phosphoprotein complex. Nature 577, 275–279 (2020).
Gilman, M. S. A. et al. Structure of the respiratory syncytial virus polymerase complex. Cell 179, 193–204 (2019).
Abdella, R., Aggarwal, M., Okura, T., Lamb, R. A. & He, Y. Structure of a paramyxovirus polymerase complex reveals a unique methyltransferase-CTD conformation. Proc. Natl Acad. Sci. USA 117, 4931–4941 (2020).
Yuan, B. et al. Structure of the Ebola virus polymerase complex. Nature 610, 394–401 (2022).
Liang, B. Structures of the Mononegavirales polymerases. J. Virol. https://doi.org/10.1128/JVI.00175-20 (2020).
Ogino, T. & Green, T. J. RNA synthesis and capping by non-segmented negative strand RNA viral polymerases: lessons from a prototypic virus. Front. Microbiol. 10, 1490 (2019).
Asenjo, A. & Villanueva, N. Phosphorylation of the human respiratory syncytial virus P protein mediates M2-2 regulation of viral RNA synthesis, a process that involves two P proteins. Virus Res. 211, 117–125 (2016).
Saikia, P., Gopinath, M. & Shaila, M. S. Phosphorylation status of the phosphoprotein P of rinderpest virus modulates transcription and replication of the genome. Arch. Virol. 153, 615–626 (2008).
Edwards, M. R., Vogel, O. A., Mori, H., Davey, R. A. & Basler, C. F. Marburg virus VP30 is required for transcription initiation at the glycoprotein gene. mBio https://doi.org/10.1128/mbio.02243-22 (2022).
Bach, S. et al. Regulation of VP30-dependent transcription by RNA sequence and structure in the genomic Ebola virus promoter. J. Virol. https://doi.org/10.1128/JVI.02215-20 (2021).
Jacob, S. T. et al. Ebola virus disease. Nat. Rev. Dis. Primers 6, 13 (2020).
Muhlberger, E. Filovirus replication and transcription. Future Virol. 2, 205–215 (2007).
Deflube, L. R. et al. Ebolavirus polymerase uses an unconventional genome replication mechanism. Proc. Natl Acad. Sci. USA 116, 8535–8543 (2019).
Bach, S. et al. Identification and characterization of short leader and trailer RNAs synthesized by the Ebola virus RNA polymerase. PLoS Pathog. 17, e1010002 (2021).
Bach, S., Demper, J. C., Biedenkopf, N., Becker, S. & Hartmann, R. K. RNA secondary structure at the transcription start site influences EBOV transcription initiation and replication in a length- and stability-dependent manner. RNA Biol. 18, 523–536 (2021).
Bach, S., Biedenkopf, N., Grunweller, A., Becker, S. & Hartmann, R. K. Hexamer phasing governs transcription initiation in the 3’-leader of Ebola virus. RNA 26, 439–453 (2020).
Peng, Q. et al. Structural basis of SARS-CoV-2 polymerase inhibition by favipiravir. Innovation 2, 100080 (2021).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018).
Kucukelbir, A., Sigworth, F. J. & Tagare, H. D. Quantifying the local resolution of cryo-EM density maps. Nat. Methods 11, 63–65 (2014).
Pettersen, E. F. et al. UCSF chimera - a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Acknowledgements
We thank all staff at the Center for Biological Imaging, Institute of Biophysics, Chinese Academy of Science (CAS) for assistance with data collection. This study was supported by the National Key Research and Development Program of China (2021YFC2300700 to Y.S.), the Strategic Priority Research Program of CAS (XDB29010000 to Y.S. and G.F.G.) and the National Natural Science Foundation of China (81871658 and 32192452 to Y.S. and 32100119 to Q.P.). Y.S. is also partially supported by the Youth Innovation Promotion Association of CAS (Y201921).
Author information
Authors and Affiliations
Contributions
Y.S., G.F.G. and J.Q. conceived the study. B.Y., J.C., M.W. and S.G. purified the protein samples and conducted biochemical and cellular experiments. B.Y. and Q.P. prepared the cryo-EM specimens and collected data. Q.P. conducted the image processing and reconstruction. J.Q. and Q.P. built the atomic models. Y.S., G.F.G., J.Q., Q.P., B.Y. and J.C. analysed the structures. Q.P., B.Y., J.Q., G.F.G. and Y.S. wrote the manuscript. All authors participated in the discussion and manuscript editing. Q.P., B.Y. and J.C. contributed equally to this work. Y.S. supervised all of the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Julien Lescar, Ming Luo, Matthias Wolf and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
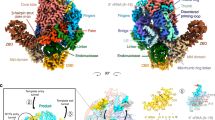
Extended Data Fig. 1 Image processing of EBOV L-VP35-RNA complex.
The density for VP35 was much weak during image processing, and we combined all particles for reconstruction to obtain a better RNA density (EM map1). And then, we reextracted the refined particles and recentered them onto the center of mass of VP35 OD for signal subtraction and another 3D-classification without alignment. Among the eight classes, we found two good classes with different orientations relative to the polymerase. These two classes were reconstructed separately and denoted as conformation 1 and conformation 2.
Extended Data Fig. 2 Cryo-EM analysis of EBOV L-VP35-RNA complex.
(a) A representative micrograph of EBOV L-VP35-RNA complex (one out of 6,392 micrographs). (b) Typical 2D class average images of EBOV L-VP35-RNA complex. (c-e) Euler angle distribution of EBOV L-VP35-RNA complex in different conformations. (f-h) Fourier shell correlation (FSC) curves of EBOV L-VP35-RNA complex in different conformations. (i) EM densities and atomic models of the RNA and key structural elements.
Extended Data Fig. 3 Conformational changes of VP35 upon RNA binding.
(a) Overlay of EBOV L-VP35-RNA complex structures in conformation 1 and conformation 2. The oligomerization domain (OD) of VP35 could rotate with certain flexibility. (b) The top view of OD in different conformations. (c) Comparison of EBOV L-VP35 in apo and RNA binding states.
Extended Data Fig. 4 The back-priming model of EBOV polymerase.
The back-priming model of EBOV polymerase using wildtype (a) or G7C mutant (b) leader sequence.
Extended Data Fig. 5 Effects of G7 mutations on RNP activity in the replicon cell.
(a) The green fluorescence intensities in replicon cell using wildtype minigenome or mutated minigenomes. (b) Protein expression levels of NP, VP35 and VP30 were determined by western blot assay. Tubulin was used as a loading control. (c) The transcriptional levels of L protein in different groups. The results represented as mean values ± s.d. from three independent experiments (n = 3). Statistical significance was determined by one-way ANOVA test, and the exact P values were indicated in (c).
Extended Data Fig. 6 The purification of wildtype and mutant EBOV L-VP35 proteins.
(a-g) Size-exclusion chromatography and SDS-PAGE profiles of wildtype and mutant EBOV L-VP35 proteins. All data shown were representative of three independent experiments (n = 3), and the uncropped gel images were shown in Supplementary Fig. 1.
Extended Data Fig. 7 Effects of L mutations on RNP activity in replicon cells.
(a) The green fluorescence intensities of replicon cells for different groups. (b) Protein expression levels of NP, VP35 and VP30 in the replicon cells for different groups. Tubulin was used as a loading control. (c) The transcriptional levels of L protein for different groups. The results represented as mean values ± s.d. from three independent experiments (n = 3). Statistical significance was determined by one-way ANOVA test, and the exact P values were indicated in (c).
Extended Data Fig. 8 The mutagenesis effect of leader RNA on the de novo initiation of viral replication.
(a) The sequence alignment of leader RNA regions of different filoviruses. (b-d) The effects of G1 (b), C2 (c) and U6 (d) mutations on the L-VP35 de novo replication activity. (e) The influence of U6 mutations on the interaction with the polymerase. The distance was indicated by a black dashed line. All data shown were representative of three independent experiments (n = 3), and the uncropped autoradiograph images were shown in Supplementary Fig. 1.
Extended Data Fig. 9 Comparison of the structures of different viral polymerases in the pre-initiation state.
(a) The EBOV polymerase in the pre-initiation state. The distinctive conformation of 3’ promoter RNA could facilitate its tight binding with L polymerase and stabilize the complex in pre-initiation state without forming duplex with the 5’ promoter RNA. (b-c) The IAV (b) (PDB ID: 6T0N) and LASV (c) (PDB ID: 7OJL) polymerases in pre-initiation state. For polymerases of sNSVs including IAV and LASV, the 3’ promoter RNA could not bind to the polymerase alone in pre-initiation conformation, and it needs to form base pairs with 5’ promoter which firmly binds to the polymerase through a hook structure.
Extended Data Fig. 10 Model of conformational transition from pre-initiation state to elongation state.
(a) The structure of EBOV L-VP35 (state 2) determined in our previous work22. The density for template RNA is much weak mainly due to its flexibility and less specific inter/intramolecular interactions, and therefore we failed to build the atomic model of template RNA. However, based on the structure of EBOV L-VP35-RNA in pre-initiation state determined here, several nucleotides could be modeled. (b) Close-up view of the density and atomic model of the template RNA shown in (a). (c) Comparison of EBOV L-VP35 structures in non-initiation state and pre-initiation state. The majority of both structures could be overlaid well, except the tip of a loop in NTD. (d) Close-up view of the structural difference for EBOV L-VP35 in non-initiation and pre-initiation states. (e)The models of EBOV L-VP35 polymerase in different states. The priming loop of EBOV L-VP35 polymerase was much flexible in apo (I) and pre-initiation state (II), and it should insert into the polymerase active site and act as a buttress for de novo initiation of replication (III) and then retract into the PRNTase domain to leave enough space for dsRNA elongation (IV). The unbuilt priming loop was indicated as green dashed line. The incoming GTP in the polymerase active site for first dinucleotide synthesis was modelled based on the structure of bacteriophage phi6 RNA polymerase in initiation state (PDB: 1H10). The dsRNA in elongation state was modelled based on the structure of rotavirus polymerase complex (PDB: 6OGZ).
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peng, Q., Yuan, B., Cheng, J. et al. Molecular mechanism of de novo replication by the Ebola virus polymerase. Nature 622, 603–610 (2023). https://doi.org/10.1038/s41586-023-06608-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06608-1
This article is cited by
-
Structural basis for dimerization of a paramyxovirus polymerase complex
Nature Communications (2024)
-
Structures of the promoter-bound respiratory syncytial virus polymerase
Nature (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.