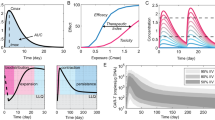
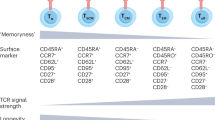
Abstract
Compliance with Food and Drug Administration regulations relating to initiating early phase clinical trials of new cellular therapy products often presents a hurdle to new investigators. One of the biggest obstacles is the requirement to manufacture the therapeutic products under current Good Manufacturing Practices—a system that is usually poorly understood by both basic researchers and clinicians. This article reviews the major points that must be addressed when manufacturing genetically modified T cells for therapeutic use.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Kakarla S, Gottschalk S . CAR T cells for solid tumors: armed and ready to go? Cancer J 2014; 20: 151–155.
Jena B, Moyes JS, Huls H, Cooper LJ . Driving CAR-based T-cell therapy to success. Curr Hematol Malig Rep 2014; 9: 50–56.
Food and Drug Administration Center for Biologics Evaluation and Research. Guidance for Human Somatic Cell Therapy and Gene Therapy. March 1998. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/ucm081670.pdf.
Human Cells, Tissues and Cellular and Tissue-based Products. Title 21 Code of Federal Regulations, Part 1271. 2014.
Current Good Manufacturing Practices in Manufacturing, Processing, Packing or Holding of Drugs. Title 21 Code of Federal Regulations, Parts 210 and 211. 2014.
Guidance for FDA Reviewers and Sponsors: Content and Review of Chemistry, Manufacturing, and Control (CMC) Information for Human Somatic Cell Therapy Investigational New Drug Applications (INDs). 2008http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Xenotransplantation/ucm074131.htm.
Guidance for FDA Reviewers and Sponsors: Content and Review of Chemistry, Manufacturing, and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications (INDs). 2008http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Xenotransplantation/ucm092705.pdf.
Considerations for the Design of Early-Phase Clinical Trials of Cellular and Gene Therapy Products. 2013http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/UCM359073.pdf.
cGMP for Phase 1 Investigational Drugs. 2008 http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070273.pdf.
Schambach A, Zychlinski D, Ehrnstroem B, Baum C . Biosafety features of lentiviral vectors. Hum Gene Ther 2013; 24: 132–142.
Anson DA . The use of retroviral vectors for gene therapy – what are the risks? Genet Vaccines 2004; 2: 9.
Singh H, Huls H, Kebriaei P, Cooper LJN . A new approach to gene therapy using sleeping beauty to genetically modify clinical-grade T cells to target CD19. Immunol Rev 2014; 257: 181–190.
Segura MM, Garnier A, Durocher Y, Coelho H, Kamen A . Production of lentiviral vectors by large-scale transient transfection of suspension cultures and affinity chromatography purification. Biotechnol Bioeng 2007; 98: 789–799.
Eligibility Determination for Donors of Human Cells, Tissues, and Cellular and Tissue-Based Products. 2007http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/ucm072929.htm.
Tanimoto K, Muranski P, Miner S, Fujiwara H, Kajigaya S, Keyvanfar K et al. Genetically engineered fixed K562 cells: potent “off-the-shelf” antigen-presenting cells for generating virus-specific T cells. Cytotherapy 2014; 16: 135–146.
Process validation: General Principles and Practice. 2011http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070336.pdf.
Potency Tests for Cellular and Gene Therapy Products. 2011http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Xenotransplantation/ucm074131.htm.
IND Meetings for Human Drugs and Biologics. 2001http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070568.pdf.
Changes to an Approved Application: Biological Products: Human Blood and Blood Components Intended for Transfusion or for Further Manufacture. 2014http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Blood/ucm062891.pdf.
Acknowledgements
This work was supported in part by the National Heart Lung and Blood contract ‘Production Assistance for Cellular Therapy’ Contract # HHSN26820100007C, by Grant RP130256 ‘Texas Assistance for Cancer Cell Therapy (TACCT)’ from Cancer Prevention and Research Institute of Texas, and by Grant 7001-14 ‘Immunotherapy for Hematologic Malignancies’ from the Leukemia and Lymphoma Society of America.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no conflict of interest.
Rights and permissions
About this article
Cite this article
Gee, A. Manufacturing genetically modified T cells for clinical trials. Cancer Gene Ther 22, 67–71 (2015). https://doi.org/10.1038/cgt.2014.71
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2014.71