Abstract
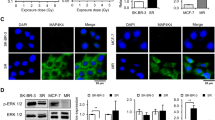
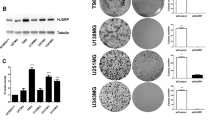
14-3-3 proteins have important roles in several cellular processes such as cell cycle progression, the DNA-damage checkpoint and apoptosis. We have shown previously that depleting 14-3-3η, a 14-3-3 isoform, enhances mitotic cell death, and that combining it with microtubule agents is more effective for anticancer therapeutics. In this study, we investigated whether depleting 14-3-3η can be combined with radiotherapy to enhance its therapeutic efficacy. We found that depleting 14-3-3η resulted in a synergistic radiosensitizing effect when combined with radiotherapy in several glioblastoma cell lines, where its specific expression and correlation of its expression level with malignancy have been reported. The radiosensitizing effect was associated with enhanced mitotic cell death by 14-3-3η depletion but not with mitotic catastrophe, which is one of the major cell death mechanisms observed in response to irradiation of most solid tumors. These results suggest that 14-3-3η may be a therapeutic target to overcome radioresistance in glioblastoma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Aitken A . 14-3-3 proteins: a historic overview. Semin Cancer Biol 2006; 16: 162–172.
Morrison DK . The 14-3-3 proteins: integrators of diverse signaling cues that impact cell fate and cancer development. Trends Cell Biol 2009; 19: 16–23.
Yaffe MB . How do 14-3-3 proteins work?— Gatekeeper phosphorylation and the molecular anvil hypothesis. FEBS Lett 2002; 513: 53–57.
Kruh GD . Introduction to resistance to anticancer agents. Oncogene 2003; 22: 7262–7264.
Perona R, Sanchez-Perez I . Control of oncogenesis and cancer therapy resistance. Br J Cancer 2004; 90: 573–577.
Bernier J, Hall EJ, Giaccia A . Radiation oncology: a century of achievements. Nat Rev Cancer 2004; 4: 737–747.
Noda SE, El-Jawahri A, Patel D, Lautenschlaeger T, Siedow M, Chakravarti A . Molecular advances of brain tumors in radiation oncology. Semin Radiat Oncol 2009; 19: 171–178.
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007; 114: 97–109.
Quigley MR, Post C, Ehrlich G . Some speculation on the origin of glioblastoma. Neurosurg Rev. 2007; 30: 16–20.
Lee CG, Park GY, Han YK, Lee JH, Chun SH, Park HY et al. Roles of 14-3-3eta in mitotic progression and its potential use as a therapeutic target for cancers. Oncogene 2013; 32: 1560–1569.
Eriksson D, Stigbrand T . Radiation-induced cell death mechanisms. Tumour Biol 2010; 31: 363–372.
Ha GH, Kim HS, Lee CG, Park HY, Kim EJ, Shin HJ et al. Mitotic catastrophe is the predominant response to histone acetyltransferase depletion. Cell Death Differ 2009; 16: 483–497.
Hermeking H, Lengauer C, Polyak K, He TC, Zhang L, Thiagalingam S et al. 14-3-3 sigma is a p53-regulated inhibitor of G2/M progression. Mol Cell 1997; 1: 3–11.
Yang X, Cao W, Lin H, Zhang W, Lin W, Cao L et al. Isoform-specific expression of 14-3-3 proteins in human astrocytoma. J Neurol Sci 2009; 276: 54–59.
Dutreix M, Cosset JM, Sun JS . Molecular therapy in support to radiotherapy. Mut Res 2010; 704: 182–189.
Deckbar D, Jeggo PA, Lobrich M . Understanding the limitations of radiation-induced cell cycle checkpoints. Crit Rev Biochem Mol Biol 2011; 46: 271–283.
Bucher N, Britten CD . G2 checkpoint abrogation and checkpoint kinase-1 targeting in the treatment of cancer. Br J Cancer 2008; 98: 523–528.
Castedo M, Perfettini JL, Roumier T, Andreau K, Medema R, Kroemer G . Cell death by mitotic catastrophe: a molecular definition. Oncogene 2004; 23: 2825–2837.
Acknowledgements
This study was supported by a National Research Foundation grant funded by the National R&D Program through the Dongnam Institute of Radiological & Medical Sciences (DIRAMS) funded by the Ministry of Science, ICT and Future Planning (50597-2013). We thank Dr Jung Ki Kim, Min Young Kim, and Min Su Ju for administrative support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Park, GY., Han, J., Han, Y. et al. 14-3-3 eta depletion sensitizes glioblastoma cells to irradiation due to enhanced mitotic cell death. Cancer Gene Ther 21, 158–163 (2014). https://doi.org/10.1038/cgt.2014.11
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2014.11
This article is cited by
-
Significant reductions in apoptosis-related proteins (HSPA6, HSPA8, ITGB3, YWHAH, and PRDX6) are involved in immune thrombocytopenia
Journal of Thrombosis and Thrombolysis (2021)
-
Bub1 is required for maintaining cancer stem cells in breast cancer cell lines
Scientific Reports (2015)