Abstract
Previous studies demonstrated selective inhibition of the BCR–ABL (breakpoint cluster region–Abelson murine leukemia oncogene) tyrosine kinase by RNA interference in leukemic cells. In this study, we evaluated the effect of BCR–ABL small interfering RNA (siRNA) and GFI1B siRNA silencing on chronic myeloid leukemia (CML) cells in myeloid blast crises. The GFI1B gene was mapped to chromosome 9 and is, therefore, located downstream of the BCR–ABL translocation in CML cells. Co-transfection of BCR–ABL siRNA and GFI1B siRNA dramatically decreased cell viability and significantly induced apoptosis and inhibited proliferation in K562 cells (P<0.0001) and primary advanced phase CML cells (P<0.0001) versus controls. Furthermore, combining of BCR–ABL siRNA and GFI1B siRNA significantly modified the expression of several relevant genes including Myc, MDR1, MRP1 and tyrosyl-phosphoproteins in primary CML cells. Our data suggest that silencing of both BCR–ABL siRNA and GFI1B siRNA is associated with an additive antileukemic effect against K562 cells and primary advanced CML cells, further validating these genes as attractive therapeutic targets.
Similar content being viewed by others
Introduction
Chronic myeloid leukemia (CML) is a relatively well-differentiated myeloproliferative disorder originating from transformed hematopoietic stem cells. It follows a fairly benign course for several years (chronic phase) before transforming into the more aggressive accelerated phase and life-threatening blast crisis (BC). CML is characterized by the Philadelphia chromosome, which results in the expression of the BCR–ABL fusion gene, and is derived from the fusion of the cellular breakpoint cluster region (BCR) gene and the Abelson murine leukemia oncogene (ABL). The expression of these consistent molecular changes has been shown to be necessary and sufficient for the transformed phenotype of CML cells.1, 2 The BCR–ABL protein activates multiple signaling pathways, including the RAS/MEK/extracellular signal-regulated kinase 1 and 2/phosphatidylinositol 3-kinase/Akt, NF-κB and signal transducer and activator of transcription protein pathways.3, 4 BCR–ABL-mediated altered expression of these pathways leads to CML progression. Strategies for targeting cellular structures of neoplastic cells include the use of low-molecular weight pharmacologically active compounds such as tyrosine kinase inhibitors (TKIs), large molecules such as antibodies, and the application of nucleic acid-based inhibitors of gene expression, that is, antisense oligonucleotides, ribozymes, DNAzymes and RNA molecules mediating RNA interference (RNAi).5 RNAi represents an evolutionarily conserved cellular mechanism that mediates sequence-specific post-transcriptional gene silencing initiated by double-stranded RNA. Small interfering RNAs (siRNA) are the mediators of mRNA degradation in the process of RNAi.6 We demonstrated that combined transfection of CML cell lines and cells of CML patients with Wilms’ tumor gene (WT1) siRNA and BCR–ABL siRNA leads to increased inhibition of proliferation and induced apoptosis compared with transfection with BCR–ABL siRNA or WT1 siRNA alone.7 We also showed that silencing by BCR–ABL siRNA combined with TKIs such as imatinib or nilotinib may be associated with an additive activity against TKI-sensitive and resistant BCR–ABL+ CML cells, and might represent an alternative approach to overcome TKI resistance due to BCR–ABL mutations.8
Growth factor independent-1B is a transcription factor with six C2H2 zinc fingers and one unique N-terminal SNAG (Snail/Gfi-1) transcriptional repressor domain. The GFI1B gene was mapped to chromosome 9q34.13 and is, therefore, located downstream of the BCR–ABL translocation in CML cells. Growth factor independent-1B signaling is important for commitment and maturation of hematopoietic cell populations.9, 10 GFI1B as target for gene silencing was chosen because it is aberrantly overexpressed in leukemias, including a high overexpression in erythropoietic and megakaryocytic malignancies, advanced CML, and in their corresponding cell lines, especially in K562 cells, a cell line that is derived from CML cells in myeloid BC. Growth factor independent-1B is much less expressed in normal hematopoietic progenitor cells than in leukemic cells, suggesting that silencing of this gene might interfere with proliferation and survival of leukemic cells.11, 12
We evaluated the antileukemic effects of BCR–ABL silencing in advanced or BC leukemic cells, and performed studies to assess if this effect can be augmented by the additional application of siRNA directed against the GFI1B gene. In order to elucidate the function of the GFI1B gene in advanced leukemia, we profiled gene expression in advanced leukemic cells in response to siRNA treatment.
Materials and methods
Cell culture
K562 cells, CML in myeloid blast crises, were grown in RPMI 1640 medium (Invitrogen, Karlsruhe, Germany) supplemented with 10% fetal bovine serum as described13 and were maintained at 37 °C in a humidified incubator with 5% CO2.
Cells from patients
Peripheral blood or bone marrow from patients with advanced phase CML was used. Informed consent was obtained before the procedure.
RNA purification
RNA was isolated using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions.
Quantitative real-time PCR
We quantified BCR–ABL, GFI1B, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), c-Myc, MDR1, MPR1 and p21CIP1/WAF1 mRNA levels by real-time reverse transcription-polymerase chain reaction (RT-PCR) using the Lightcycler (Roche Applied Science, Mannheim, Germany) or the ABI PRISM 7000 Sequence Detection System (PE Applied Biosystems, Darmstadt, Germany) device and software. Primer and hybridization probes for RT-PCR for BCR–ABL, GFI1B, MDR1 and GAPDH have been previously published.10, 11, 14 For p21CIP1/WAF1, c-Myc and MPR1 we used the primers and hybridization probes as published earlier.15, 16, 17 Quantification of RNA transcript expression was normalized determining the ratio between expression levels of targets and GAPDH.
siRNA transfection
For in vitro transfection with GFI1B siRNA, a total of 175 pM GFI1B siRNA (purchased from Qiagen, No. AF 081946, sense 5′-CAGCCCUGUCCUUAGCACUDTDT-3′ and antisense 5′-AGUGCUAAGGACAGGGCUGDTDT-3′sequences) was added to treated cells and transfection was performed as previously described.8, 13 Briefly, in vitro transfections with siRNA were performed in 24-well plates using the DOTAP liposomal transfection reagent (1 × 105 cells per well) (Roche Applied Science, Indianapolis, IN, USA) following the manufacturer’s protocol. Sequences of siRNA directed against the BCR–ABL transcript were published previously by Scherr et al.18 The BCR–ABL siRNA was also purchased by Qiagen and transfection with 54 pM BCR–ABL siRNA was performed as described for GFI1B siRNA. As control, we used two or more non-silencing siRNA (mismatched or scrambled siRNA) from Qiagen.
Growth inhibition assay
Cells were cultured in 96-well plates at a concentration of 5000 cells per well and left to recover. The number of viable cells was quantitatively estimated by a colorimetric assay using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). MTT (10 μl of 5 mg ml−1 solution, Sigma Chemical, Hamburg, Germany) was added to each well of the titration plate and incubated for 4 h at 37 °C. The cells were then solubilized by the addition of dimethyl sulfoxide (40 μl per well) and incubated for 60 min at 37 °C. The absorbance of each well determined in an enzyme-linked immunosorbent assay plate reader using an activation wavelength of 570 nm and a reference wavelength of 630 nm. The percentage of viable cells was determined by comparison with untreated control cells.
Terminal transferase deoxyuridine triphosphate nick-end labeling assay
Apoptotic cells were determined using the in situ cell Death Detection kit from Roche Molecular Diagnostics (Mannheim, Germany) following the manufacturer’s instruction. The apoptotic cells (brown staining) were counted under a microscope. The apoptotic index was defined by the percentage of brown (dark) cells among the total number of cells in each sample. Five fields with 100 cells in each were randomly counted for each sample. At least three samples with fifteen single analyses were counted.
Cell proliferation assay
Cell proliferation was determined by 5-bromo-2-deoxyuridine (BrdU) incorporation. Twenty-four hours (and up to 48 h) after transfection of siRNA, the treated cells were split into four-well chamber slides and incubated with culture medium containing BrdU for 4 h. BrdU staining was performed using the Roche Kit (Mannheim, Germany) following the manufacturer’s instructions. Analysis of proliferation was defined as the percentage of brown stained cells among the total number of cells per sample and performed likewise as the analysis of apoptotic cells.
Flow cytometry
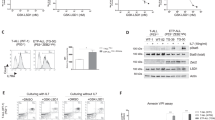
1.0–5.0 × 105 cells were labeled with antibodies for multi-color flow cytometry using fluorescein isothiocyanate, phycoerythrin or PerCP conjugated monoclonal antibodies directed against CD45, CD243 (P-gp), anti-phosphotyrosine protein (p-Tyr, PY99) and 7-AAD. For intracellular anti-phosphotyrosine protein (p-Tyr, PY99) and anti-phospho-Crkl (p-Tyr207) staining, cells were fixed with 2% paraformaldehyde for 15 min and permeabilized with 0.1% Nonidet P40 for 4 min before intracellular staining. Anti-phosphor-Crkl (p-Tyr207) was performed for 30 min at room temperature in the dark. After incubation with the primary antibody, cells were subsequently washed twice with buffer and incubated with PC5-labeled goat anti-rabbit immunoglobulin G secondary antibody. Cells were again washed and resuspended in 500 μl of PBS for flow cytometry analysis. All antibodies were obtained either from Beckmann-Coulter (Krefeld, Germany) or from Santa Cruz Biotechnology (Heidelberg, Germany). Nonspecific binding was corrected with isotype-matched controls. Flow cytometric data were acquired using a four-color Epics XL AF 14075 flow cytometer with Expo 32 ADC software (Coulter).
Statistics
Values are presented as mean±s.d. Variations in data between the different groups were tested either by a two-tailed unpaired t-test or a Mann–Whitney U-test using the SPSS 11.5 program (SPSS Inc., Chicago, IL, USA).
Results
GFI1B expression in primary CML, K562 cells and hematopoietic progenitor cells
Eighteen bone marrow samples from patients with CML in different stages and two CD34+-enriched hematopoietic progenitor samples from healthy volunteers were evaluated for their GFI1B expression measured by real-time RT-PCR and normalized to the housekeeping gene GAPDH (quotient of GFI1B/GAPDH). High levels of mRNA GFI1B were observed in CML patients in chronic phase (P<0.01 compared with CD34+-enriched hematopoietic progenitor controls). At least 35-fold increased GFI1B expression was found in CML patients with advanced phase (P<0.01) and 75-fold increased GFI1B expression in CML patients in BC (P<0.04) compared with the mean GFI1B expression in hematopoietic progenitor cells of healthy volunteers. Consistent with these observations, GFI1B expression was also increased in K562 cells. However, no significant differences in GFI1B expression were found in CD34-depleted hematopoietic progenitor samples compared with CD34+-enriched hematopoietic progenitor samples from healthy volunteers. GFI1B mRNA expression in patients with CML in advanced phase and in patients with CML in BC was strongly increased compared with patients with CML in chronic phase (58.2±13.5%, P<0.02 and 122.9±52.1%, P<0.04 versus 36.7±13.6% in CML, chronic phase), as seen in Table 1.
To analyze the efficiency of RNAi delivery, we evaluated the transfection rate in six samples of primary CML cells and in K562 cells using fluorescently marked non-silencing siRNA. Twenty-four hours after transfection, the number of transfected cells was evaluated using fluorescence microscopy counting 5 × 100 cells. We found a mean transfection rate of 45.8±12.8% in primary advanced CML cells and a mean transfection rate of 69.4±5.7% in K562 cells.
Growth inhibition of K562 cells
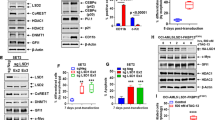
We next assessed whether transfection of BCR–ABL siRNA, GFI1B siRNA or the co-transfection with these two siRNAs affected the viability of K562 cells. Cells were transfected with constant doses (54 pM for BCR–ABL and 175 pM for GFI1B siRNA) of BCR–ABL siRNA, GFI1B siRNA, scambled siRNA or no siRNA for 48 h, harvested, and analyzed for cell viability by an MTT assay. As shown in Figure 1a, BCR–ABL siRNA, GFI1B siRNA and the combination of BCR–ABL siRNA with GFI1B siRNA significantly decreased the cell viability of K562 cells. BCR–ABL siRNA or the combination of both siRNAs revealed the strongest effects, reducing the viability of K562 cells by 28.5 to 5.3% (mean) compared with controls (one and/or two scrambled siRNAs). GFI1B siRNA reduced the viability of these cells by 49.8% (mean). Mismatched controls did not inhibit cell viability even when we doubled the siRNA dose (data not shown).
Growth rate of K562 cells after transfection with BCR–ABL siRNA, GFI1B siRNA and cotreatment with both siRNAs as determined by the MTT assay. (a) Forty-eight hours after siRNA transfection, the number of viable K562 cells decreased significantly (P<0.0001). (b) Kinetics of growth inhibition of K562 cells following application of 175 pM GFI1B siRNA and 54 pM BCR–ABL siRNA or two scrambled siRNAs and/or without siRNAs is shown. Mean and s.d. are presented. The percentage of viable cells was determined by comparison with untreated or scrambled siRNA-transfected cells. BCR–ABL siRNA, breakpoint cluster region–Abelson murine leukemia oncogene small interfering RNA.
To confirm that cell growth is dependent on the timing of siRNA silencing, we next investigated the time course of effects induced by co-transfection of BCR–ABL siRNA, GFI1B siRNA and scambled siRNA (or no siRNA) in K562 cells. A significant reduction of cell growth to 21.9%±4.5 compared with the spontaneous growth rate of viable cells was found 24 h after siRNA transfection (P<0.0001). We furthermore observed a strong inhibition of cell growth to 5.3%±1.8 (P<0.0001) compared with the spontaneous cell growth rate after 48 h of co-transfection with these siRNAs. Seventy-two hours after co-transfection of BCR–ABL siRNA and GFI1B siRNA, no further reduction of cell growth was measured in K562 cells, in fact cell growth increased to 8.6%±3.2 compared with the spontaneous cell growth rate, as shown in Figure 1b.
Taken together, these data indicate that BCR–ABL siRNA, GFI1B siRNA or the co-transfection of both siRNAs inhibits growth of K562 cells.
BCR–ABL gene expression measured by real-time RT-PCR
We quantified BCR–ABL expression in K562 cells in correlation to the housekeeping gene GAPDH by real-time RT-PCR (quotient of BCR–ABL/GAPDH) and found a significant reduction of BCR–ABL mRNA levels to amounts ranging from 135.3 to 62.4% (mean) after transfection with either GFI1B siRNA or with BCR–ABL siRNA compared with controls, as shown in Table 2. Co-transfection of BCR–ABL siRNA and GFI1B siRNA accomplished a further reduction of BCR–ABL mRNA to 33.2% (mean) compared with controls (P<0.0001). The combination of both BCR–ABL siRNA and GFI1B siRNA reduced the BCR–ABL mRNA level by twofold compared with transfection with BCR–ABL siRNA alone (P<0.0001).
GFI1B gene expression measured by real-time RT-PCR
Concordantly with the inhibition of BCR–ABL gene expression, we quantified GFI1B gene expression in correlation to GAPDH by real-time RT-PCR (quotient of GFI1B/GAPDH) after transfection of BCR–ABL siRNA, GFI1B siRNA or the co-transfection of these two siRNAs. Fourty-eight hours after transfection with GFI1B siRNA, we observed a significant reduction of GFI1B mRNA levels to 32.1% (mean) compared with controls in K562 cells (P<0.0001). We found only a very mild reduction of GFI1B mRNA levels to 132.5% (mean) after BCR–ABL siRNA transfection. The combination of BCR–ABL siRNA and GFI1B siRNA strongly reduced the GFI1B mRNA level to 25.5% (mean) compared with controls (P<0.0001) (Table 2). Comparing transfection with GFI1B siRNA alone with the co-transfection of BCR–ABL siRNA and GFI1B siRNA, we found a mild reduction of GFI1B mRNA levels after transfection with both siRNAs.
Additive effects of BCR–ABL siRNA and GFI1B siRNA on induction of apoptosis and inhibition of proliferation
As expected, we saw that transfection with BCR–ABL siRNA alone or GFI1B siRNA alone inhibited the proliferation rate of K562 cells compared with controls as shown in Figure 2a (P<0.0001 for 54 pM BCR–ABL siRNA and P<0.0001 for 175 nM GFI1B siRNA versus controls). Co-transfection with BCR–ABL siRNA and GFI1B siRNA resulted in an exaggerated decrease of the proliferation rate to 9.9%±1.7 compared with controls (controls were set up to 100%). The inhibitory efficiency of the combination of BCR–ABL siRNA and GFI1B siRNA was three times higher than the one of BCR–ABL siRNA alone and fivefold increased compared with GFI1B siRNA alone (P<0.0001 for BCR–ABL siRNA and GFI1B siRNA versus BCR–ABL siRNA or GFI1B siRNA alone). Moreover, we observed an additive effect on induction of apoptosis by co-transfection of BCR–ABL siRNA and GFI1B siRNA in K562 cells. The rate of induced apoptosis increased from 1±0.1 (controls set to 1.0) to 3.3±0.2, whereas the use of either BCR–ABL siRNA alone or GFI1B siRNA alone was again less effective (BCR–ABL siRNA alone 1.8±0.2 and GFI1B siRNA alone 1.5±0.2), as shown in Figure 2b (P<0.0001 for BCR–ABL siRNA+GFI1B siRNA versus BCR–ABL siRNA or GFI1B siRNA alone).
(a) Proliferation rate of K562 cells measured by 5-bromo-2-deoxyuridine incorporation 48 h after transfection with GFI1B siRNA, BCR–ABL siRNA and cotreatment with both siRNAs. After transfection with each siRNA, the proliferation rate of K562 cells decreased significantly (P<0.0001). Mean and s.d. are presented. (b) Apoptosis rate of K562 cells measured by terminal transferase deoxyuridine triphosphate nick-end labeling assay 48 h after transfection with GFI1B siRNA, BCR–ABL siRNA and cotreatment with both siRNAs. After transfection with each siRNA, the apoptotic rate of K562 cells increased significantly (P<0.0001). Mean and s.d. are presented. BCR–ABL, breakpoint cluster region–Abelson murine leukemia oncogene; siRNA, small interfering RNA.
Taken together, these data show that BCR–ABL siRNA, GFI1B siRNA or the co-transfection of both of these siRNAs induced apoptosis and inhibited proliferation in K562 cells.
Additive effects of co-transfection with BCR–ABL siRNA and GFI1B siRNA in primary CML cells
In order to verify the above-described antileukemic effects, we assessed BCR–ABL siRNA and GFI1B siRNA transfection in primary cells obtained from four patients with BC of CML (patients with Y253F; F317L; and E255K mutation, and one patient with TKI resistant CML). We found a reduction of BCR–ABL mRNA and GFI1B mRNA amounts compared with untreated controls. The amount of BCR–ABL mRNA was significantly reduced to 20.7% (mean) 48 h after BCR–ABL siRNA transfection and to 18.5% (mean) 48 h after co-transfection with BCR–ABL and GFI1B siRNA, as shown in Figure 3. We observed an additive effect on reduction of GFI1B mRNA by co-transfection with BCR–ABL siRNA and GFI1B siRNA in primary CML cells. The GFI1B mRNA level decreased to 57.7% (mean) after GFI1B siRNA transfection, whereas the use of cotreatment with BCR–ABL siRNA and GFI1B siRNA was more effective in inhibiting GFI1B mRNA to 54.2% (mean) compared with untreated controls.
BCR–ABL and GFI1B gene expression determined by real-time RT-PCR 48 h after siRNA transfection of primary advanced CML cells. (a) Forty-eight hours after silencing with both siRNAs in primary cells, the BCR–ABL expression decreased significantly (P<0.001). (b) Forty-eight hours after silencing with both siRNAs in primary cells, the GFI1B expression decreased significantly (P<0.001). Mean and s.d. are presented. Gene expression normalized to GAPDH by real-time RT-PCR. Control was set to 100%. BCR–ABL, breakpoint cluster region–Abelson murine leukemia oncogene; CML, chronic myeloid leukemia; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; RT-PCR, reverse transcription-polymerase chain reaction; siRNA, small interfering RNA.
Taken together, these data show that co-transfection of BCR–ABL and GFI1B siRNA efficiently inhibits gene expression in advanced CML cells.
Confirmation of relevant signaling genes and variant protein levels in primary CML cells
In order to investigate the molecular effects on CML cells, we analyzed advanced CML cells with or without siRNA silencing by oligonucleotide microarray technology. After statistical analysis, relevant genes including transcription factors and leukemia-associated genes were chosen for conformation by RT-PCR (data not shown). In this series of experiments, we analyzed MDR1, MPR1, c-Myc and p21CIP1/WAF1 in primary CML cells by RT-PCR. We found strongly decreased levels of MDR1-, MPR1- and c-Myc gene expression after co-transfection with BCR–ABL siRNA and GFI1B siRNA. Levels were decreased to up to 70% compared with controls (controls were set up to 100%), as shown in Table 3. P21CIP1/WAF1 gene expression was upregulated after transfection with GFI1B siRNA and was not affected by transfection with BCR–ABL or cotreatment with GFI1B and BCR–ABL siRNAs in primary CML cells. To further verify the findings described above, we next performed flow cytometric studies on the effects on phosphotyrosine protein (p-Tyr), phosphor-Crkl (p-Crkl) and CD243 (P-gp) expression to assess signaling pathway proteins. We found a significant reduction or inhibition of p-Tyr expression to 62% and p-Crkl to 48% after co-transfection with BCR–ABL siRNA and GFI1B siRNA (controls were set to 100%). In addition, we confirmed that similar to MDR1 expression, P-gp protein (CD243) expression is significantly decreased after transfection with BCR–ABL siRNA and GFI1B siRNA in primary CML cells (P<0.0001). Comparing the effects of BCR–ABL siRNA and GFI1B siRNA transfection on MDR1 expression and P-gp protein level, we discovered that the P-gp protein is less susceptible to siRNA treatment in primary CML cells. Taken together, these data suggest that the combination of BCR–ABL siRNA and GFI1B siRNA results in enhanced inhibition of signaling gene expression and proteins.
Discussion
The discovery that siRNA could be delivered effectively to mammalian cells indicates that it might be feasible to treat leukemia by selective intervention in leukemic cell gene regulation.16, 19 There is an increasing number of in vitro and in vivo reports on RNAi-mediated silencing of BCR–ABL fusion gene.9, 10, 20 These constructs induce target-specific cleavage of BCR–ABL mRNA without affecting the expression of c-BCR or c-ABL mRNA.21 Moreover, the first report on BCR–ABL siRNA by Wilda et al.22 showed that BCR–ABL silencing was accompanied by strong induction of apoptotic cell death. The rate of induced apoptosis was even as high as that induced by 1 μM imatinib. Other investigators have confirmed these effects of BCR–ABL siRNA on CML cells.23, 24 In this study, we examined the effects of siRNA directed against BCR–ABL and/or GFI1B, a gene downstream of the BCR–ABL signaling in CML cells. We found that both GFI1B siRNA and BCR–ABL siRNA inhibit the growth of K562 cells and primary advanced CML cells in a time-dependent manner (contingent on the transfection efficiency). The response to a single siRNA dose reached a plateau with a maximal two to threefold growth reduction after siRNA transfection. It should be emphasized that the efficiency of siRNA-mediated gene silencing is affected by a combination of factors such as variation in transfection efficiency, siRNA sequences, properties of the target mRNA and others.18, 25 As postulated, we found that coadministration of GFI1B siRNA and BCR–ABL siRNA resulted in enhanced inhibition of cell growth, BCR–ABL- and GFI1B gene expression. This may be due to effective modulation by a break-point-specific siRNA or silencing of complex signal transduction pathways controlling proteolysis of potential leukemic effectors in advanced BCR–ABL-transformed cells.26, 27 In addition to regulating gene expression at the transcriptional level, it is increasingly clear that BCR–ABL and GFI1B are also involved in post-transcriptional regulation via shuttling of heterogeneous nuclear ribonucleoproteins, microRNAs or regulatory RNAs.28, 29 Recently, Schulz et al.30 identified GFI1B, using a complementary DNA library screen, as potent negative regulator of Rag (recombination-activating gene). Using the Abelson system, they demonstrated that GFI and growth factor independent-1B bind directly to a region upstream of Erag, and that the binding of these proteins is followed by changes in the chromatin structure at the Rag locus, whereas indirect inhibition is achieved through repression of the trans-activator FoxO1. Furthermore, we found additive effects of GFI1B and BCR–ABL RNAi on apoptosis and inhibition of proliferation of treated cells. The proliferation rate decreased by two- to fivefold compared with the use of GFI1B siRNA alone (P<0.0001). In addition to the inhibition of proliferation, apoptosis was increased threefold after transfection with GFI1B and BCR–ABL siRNA (P<0.0001). This suggests that GFI1B and BCR–ABL siRNA affects leukemic cells by modulating or transforming genes via different pathways promising additive selective antitumor activity. Recently, Ohba et al.31 demonstrated by microarray analysis of K562 cells that there is crosstalk between siRNA interference and BCR–ABL oncogeny, and found that the expression ∼250 genes were changed. RNAi of BCR–ABL was accompanied by decreased expression of various protooncogenes, growth factors, factors related to kinase activity and factors related directly to cell proliferation. Several factors responsible for the development of apoptosis increased, including signal transducer and activator of transcription-induced signal transducer and activator of transcription inhibitors and apoptosis-related RNA-binding protein. The use of several siRNAs to induce additive effects toward target cells has already been described in other systems.9, 10 In experiments focused on cell cycle analysis, regulatory RNA and apoptotic responses induced by RNAi with cotreatment of GFI1B siRNA and BCR–ABL siRNA seemed to be fluctuating. There are several pharmacological mechanisms by which CML cells develop resistance to TKIs or other treatments, such as increased drug-efflux-related surface molecules including MDR1, or aberrant regulation of signal transduction.32 Of note, our real-time RT-PCR data show a highly significant reduction of MDR1, MPR1 and c-Myc expression of >40–70% after co-transfection with GFI1B siRNA and BCR–ABL siRNA in advanced phase CML cells. Myc overexpression during CML progression is consistent in CML cells during BC, where c-Myc promotes genomic instability and differentiation arrest and correlates with poor responses to imatinib. It is also conceivable that clones with high c-Myc expression are selected during CML progression and c-Myc high cells are more prone to progress to blast phase.33 In addition to impaired drug-efflux-related surface molecules, the tyrosyl-phosphoprotein proteins p-Crkl and p-Tyr were significantly reduced up to 40% after co-transfection with both siRNAs. This strongly suggests that dual inhibition of BCR–ABL by RNAi additively enhances cytotoxicity and apoptosis in advanced leukemia cells.
This study demonstrates that both BCR–ABL siRNA and GFI1B siRNA are able to induce apoptosis and inhibition of proliferation of leukemic cells. Moreover, combining GFI1B-specific siRNA with BCR–ABL-specific siRNA has additive beneficial effects and may reduce overexpression of c-Myc and MDR1 genes. We conclude that siRNA-based strategies, including the use of GFI1B-specific siRNA should have true potential for the development of innovative treatment options for patients with advanced leukemia.
References
Deininger MW, Goldman JM, Melo JV . The molecular biology of chronic myeloid leukemia. Blood 2000; 96: 3343–3356.
Goldmann JM, Melo JV . Chronic myeloid leukemia - advances in biology and new approaches to treatment. N Engl J Med 2003; 349: 1451–1461.
Radich JP, Dai H, Mao M, Oehler V, Schelter J, Druker B et al. Gene expression changes associated with progression and response in chronic myeloid leukemia. Proc Natl Acad Sci USA 2006; 103: 2794–2799.
Simanovsky M, Berlinsky S, Sinai P, Leiba M, Nagler A, Galski H . Phenotypic and gene expression diversity of malignant cells in human blast crisis chronic myeloid leukemia. Differentiation 2008; 76: 908–922.
Lage H . Potential applications of RNA interference technology in the treatment of cancer. Future Oncol 2005; 1: 103–113.
Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T . Duplexes of 21- nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001; 411: 494–498.
Elmaagacli AH, Koldehoff M, Peceny R, Klein-Hitpass L, Ottinger H, Beelen DW et al. WT1 and BCR-ABL specific small interfering RNA have additive effects in the induction of apoptosis in leukemic cells. Haematologica 2005; 90: 326–334.
Koldehoff M, Kordelas L, Beelen DW, Elmaagacli AH . Small interfering RNA against BCR-ABL transcripts sensitize mutated T315I cells to nilotinib. Haematologica 2010; 95: 388–397.
Tong B, Grimes HL, Ty Yang, Bear SE, Qin Z, Du K et al. The GFI1B proto-oncoprotein represses p21WAF1 and inhibits myloid cell differentation. Mol Cell Bio 1998; 18: 2462–2473.
Rodel B, Wagner T, Zornig M, Niessing J, Moroy T . The human homologue (GFI1B) of the chicken GFI gene maps to chromosome 9q34.13-A locus frequently altered in hematopoietic diseases. Genomics 1998; 54: 580–582.
Elmaagacli AH, Koldehoff M, Zakrzewski JL, Steckel NK, Ottinger H, Beelen DW . Growth factor-independent 1B gene (GFI1B) is overexpressed in erythropoietic and megakaryocytic malignancies and increases their proliferation rate. Br J Haematol 2007; 136: 212–219.
Vassen L, Khandanpour C, Ebeling P, van der Reijden BA, Jansen JH, Mahlmann S et al. Growth factor independent 1b (Gfi1b) and a new splice variant of Gfi1b are highly expressed in patients with acute and chronic leukemia. Int J Hematol 2009; 89: 422–430.
Koldehoff M, Zakrzewski JL, Klein-Hitpass L, Beelen DW, Elmaagacli AH . Gene profiling of growth factor independence 1B gene (GFI1B) in leukemic cells. Int J Hematol 2008; 87: 39–47.
Elmaagacli AH, Freist A, Hahn M, Opalka B, Seeber S, Schaefer SW et al. Estimating the relapse stage in chronic myeloid leukaemia patients after allogeneic stem cell transplantation by the amount of BCR-ABL fusion transcripts detected using a new real-time polymerase chain reaction method. Br J Haematol 2001; 113: 1072–1075.
Specht K, Richter T, Muller U, Walch A, Werner M, Hofler H . Quantitative gene expression analysis in microdissected archival formalin-fixed and paraffin-embedded tumor tissue. Am J Pathol 2001; 158: 419–429.
Larramendy ML, Niini T, Elonen E, Nagy B, Ollila J, Vihinen M et al. Overexpression of translocation-associated fusion genes of FGFRI, MYC, NPMI, and DEK, but absence of the translocations in acute myeloid leukemia. A microarray analysis. Haematologica 2002; 87: 569–577.
Kourti M, Vavatsi N, Gombakis N, Sidi V, Tzimagiorgis G, Papageorgiou T et al. Expression of multidrug resistance 1 (MDR1), multidrug resistance-related protein 1 (MRP1), lung resistance protein (LRP), and breast cancer resistance protein (BCRP) genes and clinical outcome in childhood acute lymphoblastic leukemia. Int J Hematol 2007; 86: 166–173.
Scherr M, Battmer K, Winkler T, Heidenreich O, Ganser A, Eder M . Specific inhibition of BCR-ABL gene expression by small interfering RNA. Blood 2003; 101: 1566–1569.
Novina CD, Sharp PA . The RNAi revolution. Nature 2004; 430: 161–164.
Koldehoff M, Steckel NK, Beelen DW, Elmaagacli AH . Therapeutic application of small interfering RNA directed against BCR-ABL transcripts to a patient with imatinib-resistant chronic myeloid leukaemia. Clin Exp Med 2007; 7: 47–55.
Wohlbold L, van der Kuip H, Miething C, Vornlocher HP, Knabbe C, Duyster J et al. Inhibition of BCR-ABL gene expression by small interfering RNA sensitizes for imatinib mesylate (STI571). Blood 2003; 102: 2236–2269.
Wilda M, Fuchs U, Wössmann W, Borkhardt A . Killing of leukemic cells with a BCR/ABL fusion gene by RNA interference. Oncogene 2002; 21: 5716–5724.
Merkerova M, Klamova H, Brdicka R, Bruchova H . Targeting of gene expression by siRNA in CML primary cells. Mol Biol Rep 2007; 34: 27–33.
Withey JM, Marley SB, Kaeda J, Harvey AJ, Crompton MR, Gordon MY . Targeting primary human leukaemia cells with RNA interference: BCR-ABL targeting inhibits myeloid progenitor self-renewal in chronic myeloid leukaemia cells. Br J Haematol 2005; 129: 377–380.
Pei Y, Tuschl T . On the art of identifying effective and specific siRNAs. Nat Methods 2006; 3: 670–676.
Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of BCR-ABL positive cells. Nat Med 1996; 2: 561–566.
Perrotti D, Jamieson C, Goldman J, Skorski T . Chronic myeloid leukemia: mechanisms of blastic transformation. J Clin Invest 2010; 120: 2254–2264.
Perrotti D, Calabretta B . Post-transcriptional mechanisms in BCR/ABL leukemogenesis: role of shuttling RNA-binding proteins. Oncogene 2002; 21: 8577–8583.
Rumpold H, Webersinke G . Molecular pathogenesis of Philadelphia-positive chronic myeloid leukemia - is it all BCR-ABL? Curr Cancer Drug Targets 2011; 11: 3–19.
Schulz D, Vassen L, Chow KT, McWhirter SM, Amin RH, Möröy T et al. Gfi1b negatively regulates Rag expression directly and via the repression of FoxO1. J Exp Med 2012; 209: 187–199.
Ohba H, Zhelev Z, Bakalova R, Ewis A, Omori T, Ishikawa M et al. Inhibition of BCR-ABL and/or c-abl gene expression by small interfering, double-stranded RNAs: cross-talk with cell proliferation factors and other oncogenes. Cancer 2004; 101: 1390–1403.
Crews LA, Jamieson CH . Chronic myeloid leukemia stem cell biology. Curr Hematol Malig Rep 2012; 7: 125–132.
Albajar M, Gómez-Casares MT, Llorca J, Mauleon I, Vaqué JP, Acosta JC et al. MYC in chronic myeloid leukemia: induction of aberrant DNA synthesis and association with poor response to imatinib. Mol Cancer Res 2011; 9: 564–576.
Acknowledgements
We thank Silke Gottwald, Melanie Kroll and Christiane Schary for their excellent technical performance of the PCR analyses and siRNA experiments. We would also like to thank Martina Franke and Ursula Hill for their flow cytometric analyses. This work was supported in part by grants of the Förderverein Essener Tumorklinik e.V. and the Kulturstiftung Essen e.V.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Author contributions
MK designed, performed and analyzed research and wrote the manuscript. JLZ contributed in writing of the paper. DWB and AHE participated in coordination of the study, and funded the study.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Koldehoff, M., Zakrzewski, J., Beelen, D. et al. Additive antileukemia effects by GFI1B- and BCR–ABL-specific siRNA in advanced phase chronic myeloid leukemic cells. Cancer Gene Ther 20, 421–427 (2013). https://doi.org/10.1038/cgt.2013.31
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2013.31
Keywords
This article is cited by
-
Modified dendritic cell-derived exosomes activate both NK cells and T cells through the NKG2D/NKG2D-L pathway to kill CML cells with or without T315I mutation
Experimental Hematology & Oncology (2022)
-
GFI1B acts as a metabolic regulator in hematopoiesis and acute myeloid leukemia
Leukemia (2022)
-
Exosomes derived from siRNA against GRP78 modified bone-marrow-derived mesenchymal stem cells suppress Sorafenib resistance in hepatocellular carcinoma
Journal of Nanobiotechnology (2018)
-
Progress in RNAi-mediated Molecular Therapy of Acute and Chronic Myeloid Leukemia
Molecular Therapy - Nucleic Acids (2015)
-
New horizons for cancer gene therapy
Cancer Gene Therapy (2014)